New publications
Preparations
Drugs that prevent thrombosis and improve blood rheology

In the prevention of the formation of multiple microthrombi during shock and their destruction, various pharmacological approaches may occur that use drugs that prevent thrombosis and improve blood rheology:
- elimination of systemic hemodynamics and microcirculation disorders with the help of vasoactive and inotropic agents;
- measures to improve blood rheology with the help of rational infusion therapy and drugs that restore the elasticity of erythrocyte membranes (trental or pentoxifylline);
- prevention of platelet aggregation and the formation of initial "white" thrombi in small arterial vessels, followed by the launch of a coagulation cascade;
- inhibition of thrombus formation after inclusion of the systemic coagulation cascade;
- activation of fibrinolysis to dissolve freshly formed thrombi (fibrinolysin, streptokinase, streptodedesis, urokinase, etc.) or, on the contrary, inhibition of fibrinolysis during its generalization in some patients with traumatic shock and sepsis (aminocaproic acid, ambene, countercrack, etc.).
Most of these approaches are traditional, well developed in the practice of treating shock, have their own hemorheological indications and are specified in the relevant chapters. Therefore, in this section it is worthwhile to consider the general approach to the prevention of thrombus formation in shock with the help of pharmacological agents that influence the prophase of blood coagulation. It is this level of prevention of coagulation complications - the occurrence, formation and growth of "white arterial blood clots" - which attracts the greatest attention of researchers.
Varied and often multidirectional disorders of blood coagulation with worsening of its rheology are characteristic for different types of shock. The most characteristic for septic, endotoxic, burn, traumatic and hemorrhagic shock types is the formation of multiple microthrombi in the smallest vessels, caused by systemic hemodynamics disorders, vasospasm and microcirculation disorders, blood thickening, slimming, decreased erythrocyte membrane elasticity, and numerous common and local factors autokoids) that initiate local changes in coagulation hemostasis and the inclusion of the prophase of blood coagulation.
In the schematic (shortened) form the initial stage of hemocoagulation and the mechanism of local hemocoagulation homeostasis is presented as follows.
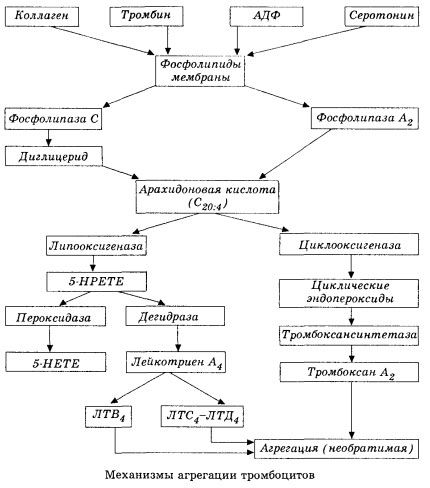
It starts with the activation of membrane phospholipase A2 as a result of the impact of a number of damaging factors (direct damage to membranes, hypoxia, lipid peroxidation, the effect of endogenous chemical factors, etc.). As a result of the cleavage of membrane phospholipids, non-esterified fatty acids with a long chain are released, of which the most important is the original substrate arachidonic acid. Its transformation (the cascade of arachidonic acid) proceeds through lipoxygenase (leukotriene synthesis) and cyclooxygenase (the synthesis of prostaglandins, thromboxanes, prostacyclin) by pathways.
Formed leukotrienes (B4, C4, E4, D4, etc.) - substances with extremely high biological activity, including slow-reacting substance of anaphylaxis, are important in initiating local vascular, inflammatory and immune reactions, including autoimmune processes. Leukotrienes cause disorders of microcirculation, increased blood clotting, the release of autolytic lysosomal enzymes and the release into the blood of a factor that inhibits myocardial contractility, bronchospasm.
Due to the ability to cause smooth muscle contraction, leukotrienes significantly affect systemic hemodynamics, coronary vessels and myocardium, exerting a powerful coronary-controctor and negative inotropic effect, which is accompanied by a decrease in cardiac output and plays an important role in the development of hypotension.
Decreased cardiac output and hypotensive response to the action of leukotrienes is associated with weakening of the heart muscle and limiting venous return to the heart. A significant role in limiting venous return has the ability of leukotrienes to increase the permeability of the vascular wall and cause extravasation of plasma. Leukotrienes are important in the pathogenesis of myocardial infarction.
In anaphylactic and septic (endotoxin) shock, their role seems to increase even more, as evidenced by the ability of leukotrienes to accumulate in significant amounts in plasma during allergic reactions and cause systemic blood flow changes characteristic of anaphylactic shock, as well as the protective effects of leukotriene receptor blockers and inhibitors of lipoxygenase. Development of selective blockers of leukotriene receptors is quite intensive and is a promising direction of science. In this area, a certain success has been achieved and experimentally confirmed the effectiveness of such blockers in myocardial ischemia, endotoxin and hemorrhagic shock. However, before the clinical implementation of this trend will probably take a few more years.
If blood clots form in the venous vessels with equal participation of platelets and plasma clotting factors, then in the arteries platelets are the main initiator of the process. They contain ADP, Ca2 +, serotonin, phospholipids, enzymes of the synthesis of prostaglandins and thromboxanes, thrombostenin (like the muscle actomyosin provides the contractile capacity of these cells), thrombogenic growth factor of the epithelium and muscle cells of the vessel wall and a number of other substances. Humoral regulation of platelet functions is carried out through specialized receptors of their membranes (alpha2- and beta2-adrenoreceptors, receptors for histamine and serotonin, acetylcholine, thromboxane, adenosine and a number of others). A special property of platelets is a high affinity for collagen and other subendothelial elements of the vessel wall, to non-wettable and non-depleted surfaces. This property provides the platelets with an exceptional ability to adhere (adhere) to the site of the vessel with damaged endothelium, for which damage in the face of shock is a great opportunity. At the same time, thrombocytes spread out and release pseudopodia, which can adhere to each other and to the wall of the vessels. Membrane permeability increases, and ADP, serotonin, thromboxane and some clotting factors sorbed on the platelet surface are released from platelets. These substances interact with the corresponding receptors on the membrane and with the participation of calcium ions cause aggregation (initially reversible). The process becomes self-sustaining, facilitated by the regulatory factors of humoral factors; other factors, on the contrary, can stop it and even reverse it, triggering disaggregation.
With the prevalence of thrombus-forming influences and the conditions of the phase of adhesion and reversible aggregation are replaced by the third phase - irreversible aggregation, which is carried out with the participation of thrombostenin and leads to the clot constriction; the hardening reaction of the aggregate and the constriction also occurs with the participation of Ca +, ATP and leads to the formation of a white thrombus.
The cyclooxygenase pathway of arachidonic acid transformations in platelets, vascular endothelial cells and in other tissues provides local (long half-life of metabolites is very small) coagulation homeostasis, because during this metabolism powerful pro-and antiaggregant substances are formed. The main factor that activates platelet aggregation in the cyclooxygenase chain reaction is thromboxane A2, and its no less powerful antagonist is prostacyclin produced by endothelial cells and, to a lesser extent, prostaglandins of the E and G series. Finally, platelet aggregation is strongly influenced by additional local and systemic humoral factors.
Activators and inhibitors of platelet aggregation
|
Initiators and activators of platelet aggregation |
Inhibitors of platelet aggregation |
|
Collagen |
- |
|
ADP |
Adenosine and its stabilizers |
|
Norepinephrine (via alpha2 receptors) |
Alpha-adrenergic agents |
|
Serotonin |
Antiserotonin agents |
|
Histamine |
Antihistamines |
|
Thrombin |
Heparin |
|
Ca2 + |
The antagonists of Ca2 + |
|
CGMP - its inducers (acetylcholine?) and stabilizers |
CAMP - its inducers (via beta-adrenergic receptors) and stabilizers (phosphodiesterase inhibitors) |
|
Arachidonic acid |
Dextrans, albumin |
|
Thromboxane A2 |
Prostacyclin I2 |
Pharmacological interventions in the initial phase of thrombosis in shock and acute ischemic processes in the heart and brain suggest the following possibility:
- inhibition of initial reactions (general and partial) of the cascade of arachidonic acid;
- inhibition of the partial synthesis reaction of thromboxanes;
- blockade of receptors for leukotrienes and thromboxanes in platelets, smooth muscle and other cells;
- use of substances that modulate the aggregation of platelets, ie, weakening by other ways the reaction of the latter to the action of initiating factors (collagen, thromboxane A2, leukotrienes, etc.).
The implementation of the abovementioned ways of correction of rheological blood disorders provides for the solution of the main tactical task: to protect the receptors of aggregation and adhesion of platelets from the action of activators or to suppress intracellular mechanisms for the synthesis of these receptors. Inhibition of the initial reactions of the arachidonic acid cascade can be achieved by protecting platelet receptors reacting to polymer activators with low molecular weight dextrans, whose molecules compete with fibrin, collagen, aggregated immunoglobulin (IgE), and components of the complement system.
By masking receptors on the platelet membrane and competing with large-dispersed proteins on the surface of red blood cells, low molecular weight dextrans displace them and destroy the bridges between the cells. This is due to the fact that dextrans, enveloping the vascular endothelium and the surface of the cellular elements of the blood, increase their negative charge, thereby increasing antiaggregatory properties.
Dextran
Low molecular weight dextrans reduce collagen- and ADP-induced platelet aggregation, as well as the activating effect of thrombin on platelets, inhibit the growth of the original white platelet thrombus, improve blood flow, reduce the postoperative increase in fibrinogen in the plasma, alter the structure and stability of fibrin.
Intravenous infusions of dextrans in trauma and shock not only reduce aggregation and adhesion of platelets, but also mobilize endogenous heparin, thereby contributing to the formation of a loose and slightly retractable blood clot that is readily lysed by fibrinolytic agents. The antithrombin activity of low molecular weight dextrans is attributed to their specific effect on the structure and function of the factor VIII coagulation factor. Factor VIII (antihemophilic globulin), a large molecule with a complex structure and function, takes part in the aggregation of platelets and in the stability of the clot formed. Dextrans interfere with the action of factor VIII, thereby slowing platelet aggregation and decreasing the stability of the clot.
Low molecular weight dextrans are not true anticoagulants and their corrective effect in hemorheological disorders is mainly related to hemodilution, replenishment of the circulating plasma volume and improvement of blood flow in the microcirculation system.
The ability of dextrans to improve blood flow in hemodynamic disorders (shock, blood loss) is due to a complex of factors. The appearance in the blood of high transient concentration of the polymer not only leads to "direct hemodilution", but also creates conditions for the flow of fluid into the bloodstream from the interstitial space and the subsequent balancing of the osmotic effect of dextran. As a consequence of hemodilution, the viscosity of the blood decreases, the venous influx to the heart rises and the minute volume of the heart increases. Along with these effects, dextrans form complexes with fibrinogen and have an antilipemic effect.
Thus, the anti-aggregation effect and hemodynamic effects of low molecular weight dextran contribute to a decrease in blood viscosity, which is especially important at low shear rates. Disaggregation of blood elements improves systemic blood flow and microcirculation, especially in its venous part, where the velocity gradients are the lowest. The use of low molecular weight solutions of dextrans for various types of shock, in the course of surgical treatment of injuries and their consequences, then in the postoperative period allows to prevent hypercoagulation and reduce the probability of occurrence of thrombotic process and emboli.
However, it should be noted that in some cases, infusions of dextran solutions are accompanied by anaphylactic and allergic reactions (dangerous in the presence of sensitization and anaphylactic shock). This is due to the fact that dextrans having a large molecular weight and many side chains can act as an antigen. Therefore, to establish individual sensitivity, it is recommended to pre-inject intravenously as a hapten to 20 ml of a low molecular weight dextran solution (15% solution, molecular weight 1000) and to perform infusion of the plasma substitute before the introduction of anesthesia.
Thrombin Inhibitors
Pharmacological protection of platelet receptors interacting with platelet activators can also be achieved by means competing with non-polymeric platelet activators or inhibiting them. Such agents include thrombin inhibitors (heparin and hirudin, a number of synthetic inhibitors, adrenaline antagonists), alpha receptor blockers (phentolamine, dihydroergotamine), ADP antagonists (dipyridamole, adenosine and its structural analogues, phosphocreatine), serotonin antagonists (methylshegide). Only a few of these drugs are actually used for the prevention and therapy of shock of different genesis.
Protection of intracellular mechanisms for the synthesis of protein receptors reacting with aggregation and platelet adhesion promoters and the inhibition of thromboxane synthesis processes are possible with preparations of various groups:
- inducers and stabilizers of cATP, prostacyclin and prostaglandin PgE2;
- inhibitors of phospholipase and phosphodiesterase.
Intensive development of special antiplatelet agents began relatively recently and has not yet led to reliable results. Currently, in clinical practice, antiaggregants such as acetylsalicylic acid, indomethacin, dipiridamol, sulfinpyrazone (persanthin), prostacyclin (eicoprostenone), heparin are widely used to prevent the formation of white platelet thrombi, in addition to dextran solutions.
Non-steroidal anti-inflammatory drugs
It was established that the pharmacological effects of non-steroidal anti-inflammatory drugs - acetylsalicylic acid and indomethacin, are due to their effect on the metabolism of eicosanoids (thromboxanes and prostaglandins). Almost all drugs in this group inhibit the enzyme complex, known as prostaglandin synthetase, thus providing its specific and antiplatelet effects.
Acetylsalicylic acid after intake is absorbed very quickly. Its hydrolysis product, salicylic acid, causes inhibition of platelet cyclooxygenase, as a result of which the conversion of arachidonic acid to prostaglandin 02 and, ultimately, thromboxane A2, is disrupted. Acetylsalicylic acid inhibits aggregation induced by collagen, ADP, epinephrine and serotonin. Although HGO 5 is 15 minutes, the antiplatelet effect lasts several days, which is probably due to the irreversible inhibition of prostaglandin synthesis and suppression of platelet aggregation during the entire period of their life (6-10 days). Along with the inhibition of platelet cyclooxygenase, acetylsalicylic acid at high doses inhibits the cyclooxygenase of the vascular wall and simultaneously with the suppression of the synthesis of thromboxane A2 inhibits the synthesis of prostacyclin in endothelial cells. Therefore, the appointment of acetylsalicylic acid as an antiaggregate should be in small doses (3000-5000 mg / day), which preferentially inhibit platelet aggregation.
Given that acetylsalicylic acid blocks platelet cyclooxygenase for several days, and endothelial cyclooxygenase - no more than a day, it is rational to prescribe the drug not every day, but 3-4 days later. Selection for the patient the optimal dose of acetylsalicylic acid should be carried out individually, since there is a different sensitivity of patients to the antiplatelet effect of the drug. In reactive patients, acetylsalicylic acid at a dose of 0.5 g inhibits platelet aggregation by 40-50%, in hyper-reactive ones - completely or by 80-90%, and for areactive patients, the absence of an antiaggregant effect is typical when taking the same dose of the drug.
Selective inhibitors of thromboxane synthetase are imidazole and its analogues, which do not block cyclooxygenase. Dipyridamole, used in clinical practice in the treatment of chronic ischemic heart disease as a coronary heart, similarly imidazole selectively inhibits thromboxane synthetase, preventing the synthesis of thromboxane A2. The drug and its analogs are also thought to inhibit platelet phosphodiesterase, thereby increasing the concentration of cAMP in platelets. Along with this, dipyridamole inhibits the activity of adenosine deaminase and platelet capture of adenosine, blocks the absorption of serotonin by platelets and their aggregation induced by adrenaline and collagen. There are reports of weak antiplatelet activity of the drug and its ability in low doses to increase platelet aggregation. The most reliable antiplatelet effect can be achieved by the combination of dipyridamole with acetylsalicylic acid.
Heparin
Among antithrombotic agents, one of the most effective regulators of the aggregate state of the blood is heparin, especially in its early application. Heparin has a high negative charge and is able to interact with both large and small ions and molecules (enzymes, hormones, biogenic amines, plasma proteins, etc.), so the spectrum of its biological effect is quite wide. The drug has antithrombin, antithromboplastin and antiprotrombinovoe action, prevents the transition of fibrinogen into fibrin, suppresses retraction of the clot, increases fibrinolysis.
The mechanism of anticoagulant action of heparin is rather complicated. It has now been established that anticoagulant effects of heparin are associated with potentiating the action of antithrombin III and enhancing the ability of the heparin-antithrombin III complex to rapidly inactivate most of the serine proteases of the blood coagulation system. In the antithrombotic effect of heparin, its ability to increase and maintain a high electronegative potential of intima of the vessels, which prevents platelet adhesion and the formation of platelet microthrombi, is of great importance. The most active heparin inhibits thrombus formation in the veins, thus preventing both local formation of thrombi and disseminated intravascular coagulation.
Prostacyclin and its stable analogs
Among antiplatelet agents, the most potent inhibitors of aggregation are prostacyclin and its stable analogs. The antiplatelet effect of prostacyclin is due to the stimulation of adenylate cyclase and, as a consequence, an increase in the concentration of cAMP in platelets, a decrease in thromboxane, a decrease in thromboxane A2 and blockade of its receptors. Prostacyclin is unstable and rapidly hydrolyzed to inactive products, so it is injected into the vein drip at a rate of 2 to 20 ng / kg per minute for 30-60 minutes up to 6 times per day.
Prostacyclin, along with a strong anti-aggregation effect, has a powerful vasoconstrictor and bronchodilator effect. The drug expands the vessels of the brain, heart, kidneys, skeletal muscles and mesenteric vessels. Under the influence of prostacyclin, the coronary blood flow increases, the energy supply of the myocardium increases and its oxygen demand decreases. Despite its instability in the body, a clinically beneficial effect can last for several weeks and even months. The mechanism of such a prolonged action is not yet clear.
Prostacyclin is a low-toxic drug, however, it can have side effects: facial hyperemia, headaches, lower blood pressure, abdominal pain, anorexia. Along with prostacyclin, promising inhibitors of platelet aggregation are its synthetic stable analogs (iloprost, etc.).
Drugs that improve blood viscosity
Violations of the rheological properties of blood in trauma and shock are due not only to changes in the functional activity of platelets, but also to an increase in the viscosity of the blood. The structural viscosity of blood as a complex dynamic disperse system is largely determined by the viscosity of the plasma and the ability of erythrocytes to deform. The viscosity of the plasma depends mainly on the concentration of proteins in the blood. Proteins with a small molecular weight, such as albumin, have little effect on the viscosity of the plasma, while proteins with a large molecule (fibrinogen, alpha and gamma globulins, other macromolecules) significantly increase it.
At low shear rates, adsorption on the erythrocyte surface of fibrinogen and globulin leads to the formation of bridges between neighboring cells and the formation of aggregates from erythrocytes. The rate of formation of aggregates is a complex biophysical process and depends not only on the magnitude of the shift, but also on the electrokinetic properties of erythrocytes, the concentration, mass and sorption capacity of macromolecule-aggregates, and the shape and plasticity of red blood cells.
Maintaining the shape and mechanical properties of the erythrocyte membrane requires considerable energy. It is believed that the energy produced in erythrocytes during the glycolysis process is spent on phosphorylation of the spectrin, as a result of which the secondary structure of the protein changes and interacts with the neighboring components of the inner membrane. The interaction between membrane structural proteins, spectrin and actin plays an important role in the formation of the mechanical properties of the erythrocyte membrane, in maintaining the constant surface area of the erythrocyte and its thickness for any deformation.
With violations of systemic hemodynamics and organ blood flow, an increase in the stiffness of erythrocyte membranes and the formation of erythrocyte aggregates leads to a decrease in the rate of passage of red blood cells through the capillaries, thereby violating the gas transport function of the blood. Therefore, correction of rheological disorders in blood during shock should provide, along with prevention of erythrocyte aggregation, normalization of plasma and blood viscosity, aggregation and deformation of erythrocytes.
In addition to low molecular weight dextrans, albumin solutions are one of the effective means of increasing the suspension stability of the blood. In the late period of shock, generalized aggregation of erythrocytes occurs against the background of a decrease in plasma concentrations of albumin and an increase in the concentrations of fibrinogen and globulins, especially the alpha2 fraction, lipoproteins and lipids. Under these conditions rheological effects of albumin are caused by two main factors: hemodilution and normalization of the relationships between micro- and macroglobular plasma proteins. Simultaneously, albumin binds free acids, whose labialization in trauma and shock stimulates the aggregation of cellular blood structures and intravascular coagulation and can cause fat embolism.
Anti-shock measures aimed at replenishing the volume of circulating blood, eliminating tissue hypoxia and metabolic acidosis, contribute to the normalization of the elasticity of erythrocyte membranes, since hypoxia and acidosis significantly reduce the deformability of erythrocytes. The increase in stiffness of erythrocyte membranes in shock is probably related to the inhibition of ATP synthesis in erythrocytes. In turn, a decrease in the concentration of ATP promotes an increase in the concentration of Ca2 + in erythrocytes, which, when bound to membrane proteins, increases the rigidity of the membrane.
One of the pharmacological drugs that increase the content of ATP in erythrocytes and the elasticity of erythrocyte membranes is trental (pentoxifylline), which is used in clinical practice for the treatment of ischemic disorders.
Along with a decrease in the rigidity of erythrocyte membranes, trental induces vasodilation, improves oxygenation of tissues, inhibits phosphodiesterase activity in tissues, increases cAMP concentration, and inhibits platelet aggregation.
Among other pharmacological agents that preserve the elasticity of the erythrocyte membrane, it should be noted the antagonists of Ca2 +, which limit the entry of ions into erythrocytes (flunarizine, nifedepine, etc.).
