Aortic regurgitation
Last reviewed: 23.04.2024

All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.
Aortic regurgitation is the failure of the aortic valve to close, resulting in flow from the aorta to the left ventricle during diastole. Causes include idiopathic valvular degeneration, acute rheumatic fever, endocarditis, myxomatous degeneration, congenital bicuspid aortic valve, syphilitic aortitis and connective tissue diseases or rheumatologic pathology.
Symptoms are dyspnea on exertion, orthopnea, paroxysmal night dyspnea, palpitations and chest pain. On examination, it is possible to identify a diffuse pulse wave and hunger / astrophic noise. The diagnosis is made with an objective examination and echocardiography. Treatment involves the replacement of the aortic valve and (in some cases) the administration of vasodilating drugs.
Causes of the aortic regurgitation
Aortic regurgitation (AR) may be acute or chronic. The primary causes of acute aortic regurgitation are infectious endocarditis and dissection of the ascending part of the aorta.
Mild chronic aortic regurgitation in adults is most often caused by a bicuspid or fenestrated aortic valve (2% of men and 1% of women), especially if severe diastolic hypertension is present (BP> 110 mmHg).
Moderate and severe chronic aortic regurgitation in adults is most often caused by idiopathic degeneration of aortic valves or aortic root, rheumatic fever, infective endocarditis, myxomatous degeneration or trauma.
In children, the most common cause is ventricular septal defect with aortic valve prolapse. Sometimes aortic regurgitation is caused by a seronegative spondyloarthropathy (ankylosing spondylitis, reactive arthritis, psoriatic arthritis), RA, SLE, arthritis associated with ulcerative colitis, syphilitic aortitis, osteogenesis imperfecta, an aneurysm of the thoracic aorta, aortic dissection, supravalvular aortic stenosis, Takayasu's arteritis, rupture of the sinus Valsalva, acromegaly and temporal (giant cell) arteritis. Aortic regurgitation due to myxomatous degeneration may develop in patients with Marfan syndrome or Ehlers-Danlos syndrome.
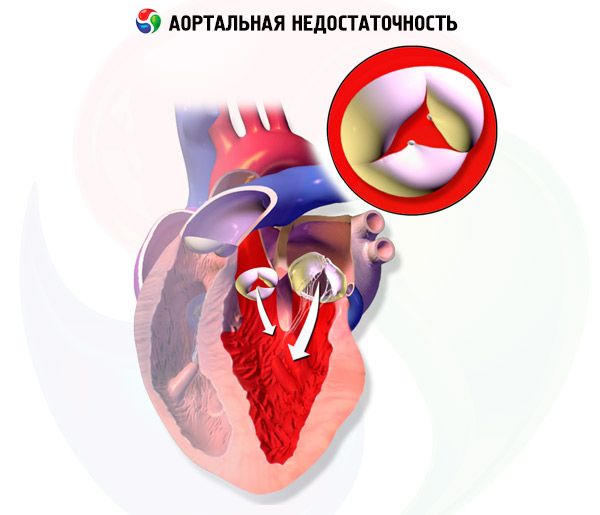
In chronic aortic regurgitation, the volume of the left ventricle and the stroke volume of the left ventricle gradually increase, since the left ventricle receives blood from the aorta to the diastole due to regurgitation, in addition to the blood from the pulmonary veins and the left atrium. Left ventricular hypertrophy compensates for an increase in its volume over several years, but ultimately decompensation develops. These changes can lead to the development of arrhythmias, heart failure (HF) or cardiogenic shock.
Symptoms of the aortic regurgitation
Acute aortic regurgitation causes symptoms of heart failure and cardiogenic shock. Chronic aortic regurgitation is usually asymptomatic for many years; progressive dyspnea on exertion, orthopnea, paroxysmal night dyspnea and palpitations develop imperceptibly. Symptoms of heart failure correlate poorly with objective indicators of left ventricular function. Chest pain (angina pectoris) occurs in approximately 5% of patients who do not have concomitant IHD, and more often this occurs at night. There may be signs of endocarditis (for example, fever, anemia, weight loss, embolism of different localization), because the pathological aortic valve is predisposed to bacterial damage.
Symptoms vary according to the severity of aortic regurgitation. As the progression of chronic diseases occurs, systolic blood pressure increases with a decrease in diastolic blood pressure, which leads to an increase in pulse pressure. Over time, the push of the left ventricle may increase, expand, increase in amplitude, shift down and sideways, with systolic collapse of the anterior left parasternal region, which creates a “swinging” movement of the left half of the chest.
In the later stages of aortic regurgitation, systolic tremor in the apex of the heart and above the carotid arteries can be detected by palpation; this is caused by a large stroke volume and low aortic diastolic pressure.
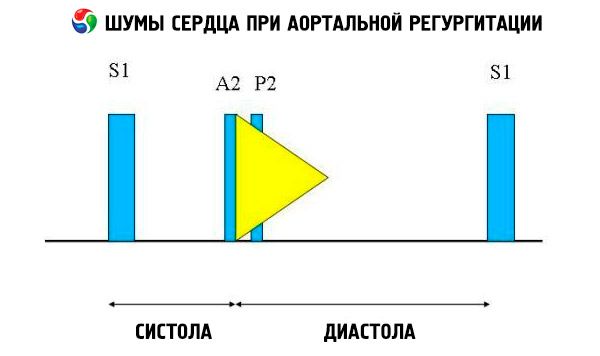
Auscultatory symptoms include normal heart tone and unsplit, loud, pointed or flapping II heart tone due to increased resistance of the elastic aorta. The aortic regurgitation noise is bright, high-frequency, diastolic, subsiding, begins shortly after the aortic component S. It is most loudly heard in the third or fourth intercostal space to the left of the sternum. Noise is heard best of all with a diaphragm stethoscope when the patient is tilted forward with breath holding on exhalation. It is enhanced by samples that increase the afterload (for example, squat, isometric handshake). If aortic regurgitation is small, noise can occur only in the early diastole. If the diastolic pressure of the left ventricle is very high, the noise becomes shorter, since the aortic pressure and the diastolic pressure of the left ventricle are equalized in the early diastole.
Other abnormal auscultatory findings include exile noise and regurgitation flow noise, ejection click shortly after S, and aortic ejection flow noise. Diastolic murmur heard in the armpit or in the middle of the left half of the chest (Cole-Cecil noise) is caused by the merging of aortic noise with III heart tone (S 3 ), which occurs due to the simultaneous filling of the left ventricle from the left atrium and aorta. Middle and late diastolic murmur, which is heard at the apex (Austin Flint noise), may be due to the rapid flow of regurgitation into the left ventricle, which causes the mitral valve to vibrate at the peak of the atrial flow; this noise is similar to diastolic murmur of mitral stenosis.
Other symptoms are rare, they have low (or unknown) sensitivity and specificity. Visible signs of the disease include rocking of the head (a symptom of Musset) and pulsation of the nail capillaries (a symptom of Quincke, better defined with slight pressure) or of a tongue (a symptom of Muller). Palpation can reveal a tense pulse with a rapid rise and fall (“beating”, “water hammer”, or a collaptoid pulse) and pulsation of the carotid arteries (Corrigen symptom), retinal arteries (Becker symptom), liver (Rosenbach symptom) or spleen (Gerhard symptom) ). Changes in blood pressure include increased systolic pressure on the legs (below the knee) at> 60 mm Hg. Art. Compared with pressure on the shoulder (Hill's symptom) and a drop in diastolic blood pressure of more than 15 mm Hg. Art. When raising a hand (symptom of Maine). Auscultatory symptoms include a rough noise that is heard in the femoral ripple region (the sound of a pistol shot, or Traube symptom), and a femoral systolic tone and diastolic murmur proximal to artery constriction (Durozier's noise).
Diagnostics of the aortic regurgitation
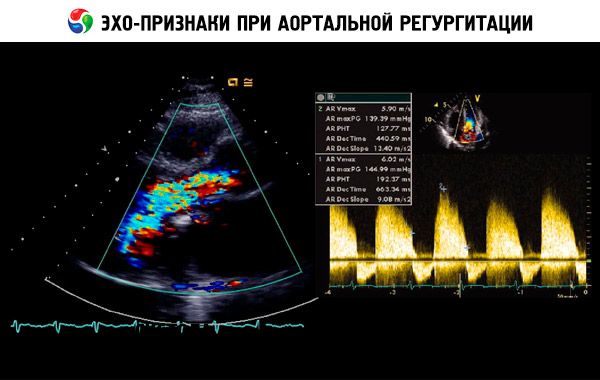
A presumptive diagnosis is made on the basis of anamnesis, an objective examination and confirmed by echocardiography. Doppler echocardiography is the method of choice for detecting and quantifying the value of the regurgitation flow. Two-dimensional echocardiography helps determine the size of the aortic root, as well as the anatomical features and functioning of the left ventricle. Of course, the left ventricle systolic volume is> 60 ml / m 2, the final systolic diameter of the left ventricle is> 50 mm, and LV LV <50% indicates decompensation. Echocardiography can also assess the severity of pulmonary hypertension secondary to left ventricular insufficiency, detect vegetation or effusion into the pericardial cavity (for example, during aortic dissection) and assess the prognosis.
Radioisotope scanning can be used to determine LV EF if the results of echocardiography border on pathology or echocardiography is technically difficult to perform.
Perform ECG and chest X-ray. The ECG may demonstrate impaired repolarization with a change (or without them) of the QRS complex, characteristic of LV hypertrophy, an increase in the left atrium, and an inversion of the T wave with ST depression in the chest leads. A chest x-ray can reveal cardiomegaly and an enlarged aortic root in patients with chronic progressive aortic regurgitation. With severe aortic regurgitation, symptoms of pulmonary edema and heart failure may occur. The exercise test helps to assess the functional reserve and the clinical manifestations of pathology in patients with identified aortic regurgitation and questionable manifestations.
Coronary angiography is usually not needed for diagnosis, but it is performed before surgery, even in the absence of angina, as approximately 20% of patients with severe AR suffer from severe coronary artery disease, which can become an indication for concomitant surgical treatment (CABG).
What do need to examine?
How to examine?
Who to contact?
Treatment of the aortic regurgitation
Treatment of acute aortic regurgitation - replacement of the aortic valve. Treatment of chronic aortic regurgitation depends on the clinical manifestations and the degree of LV dysfunction. Patients with symptoms that occur during normal daily activity or during an exercise test require replacement of the aortic valve. Patients who do not agree to surgical treatment can take vasodilators (for example, nifedipine dopital action of 30-90 mg 1 time per day or ACE inhibitors). You can also assign diuretics or nitrates to reduce preload with severe aortic regurgitation. Patients without clinical manifestations with LV EF <55%, a final systolic diameter of> 55 mm (“rule 55”) or a final diastolic diameter of> 75 mm also need surgical treatment; medication is in second place for this group of patients. Additional surgical criteria include a decrease in EF <25–29%, a ratio of end diastolic radius to myocardial wall thickness> 4.0, and a cardiac index <2.2–2.5 l / min per 1 m 2.
Patients who do not meet these criteria are subject to a thorough physical examination, echocardiography and, possibly, radioisotope angiocynography under pressure and at rest to determine LV contractility every 6-12 months.
Prophylaxis of endocarditis with antibiotics before procedures that can lead to bacteremia has been shown.
Forecast
During treatment, 10-year survival in patients with small or moderate aortic regurgitation is 80-95%. With timely valve replacement (before the development of heart failure and taking into account the criteria described below), the long-term prognosis in patients with moderate and severe aortic regurgitation is not bad. However, with severe aortic regurgitation and heart failure, the prognosis is much worse.
 [16],
[16],

