Medical expert of the article
New publications
Polycystic ovary syndrome.
Last reviewed: 04.07.2025

All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.
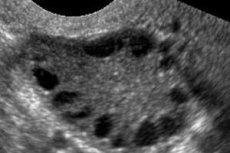
Polycystic ovary syndrome is a multifactorial heterogeneous pathology characterized by menstrual cycle disorders, chronic anovulation, hyperandrogenism, cystic changes in the ovaries, and infertility. Polycystic ovary syndrome is characterized by moderate obesity, irregular periods or amenorrhea, and symptoms of androgen excess (hirsutism, acne). The ovaries usually contain many cysts. Diagnosis is based on pregnancy tests, hormone levels, and examination to exclude a virilizing tumor. Treatment is symptomatic.
Causes polycystic ovarian syndrome
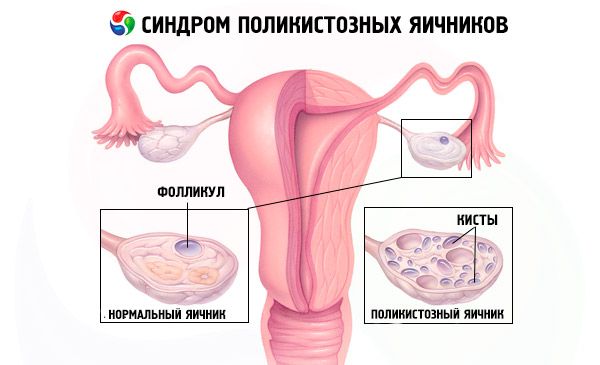
Polycystic ovary syndrome is a common endocrine pathology of the reproductive system, occurring in 5-10% of patients; it is characterized by anovulation and excess androgens of unknown etiology. The ovaries may be normal in size or enlarged, with a smooth, thickened capsule. As a rule, the ovaries contain many small, 26 mm follicular cysts; sometimes large cysts containing atretic cells are encountered. Estrogen levels increase, which leads to an increased risk of endometrial hyperplasia and, ultimately, endometrial cancer. Androgen levels are often increased, which increases the risk of metabolic syndrome and hirsutism.
 [ 13 ]
[ 13 ]
Pathogenesis
Women with polycystic ovary syndrome (PCOS) have abnormalities in androgen and estrogen metabolism, and impaired androgen synthesis. The disease is accompanied by high serum concentrations of androgen hormones such as testosterone, androstenedione, dehydroepiandrosterone sulfate and (DHEA-S). However, normal androgen levels can sometimes be determined.
PCOS is also associated with insulin resistance, hyperinsulinemia, and obesity. Hyperinsulinemia may also lead to suppression of SHBG synthesis, which in turn may enhance androgenic features.
In addition, insulin resistance in PCOS is associated with adiponectin, a hormone secreted by adipocytes that regulates lipid metabolism and blood glucose levels.
Increased androgen levels are accompanied by an increase in the stimulating effect of luteinizing hormone (LH) secreted by the anterior pituitary gland, which leads to growth of the ovarian theca cells. These cells, in turn, increase the synthesis of androgens (testosterone, androstenedione). Due to a decreased level of follicle-stimulating hormone (FSH) in relation to LH, the ovarian granular cells cannot aromatize androgens into estrogens, which leads to a decrease in estrogen levels and subsequent anovulation.
Some evidence suggests that patients have a functional impairment of cytochrome P450c17, 17-hydroxylase, which inhibits androgen biosynthesis.
Polycystic ovary syndrome is a genetically heterogeneous syndrome. Studies of family members with PCOS prove autosomal dominant inheritance. Recently, a genetic link between PCOS and obesity has been confirmed. A variant of the FTO gene (rs9939609, which predisposes to general obesity) is significantly associated with susceptibility to the development of PCOS. Polymorphisms of the 2p16 locus (2p16.3, 2p21 and 9q33.3) associated with polycystic ovary syndrome have been identified, as well as the gene encoding the receptor of luteinizing hormone (LH) and human chorionic gonadotropin (hCG).
Symptoms polycystic ovarian syndrome
Symptoms of polycystic ovary syndrome begin during puberty and decrease with time. Regular menstruation for some time after menarche excludes the diagnosis of polycystic ovary syndrome. Examination usually reveals copious cervical mucus (reflecting high estrogen levels). The diagnosis of polycystic ovary syndrome can be suspected if a woman has at least two typical symptoms (moderate obesity, hirsutism, irregular periods, or amenorrhea).
The most common combination of clinical symptoms is:
- menstrual cycle disorders (oligomenorrhea, dysfunctional uterine bleeding, secondary amenorrhea);
- anovulation;
- infertility;
- hirsutism;
- disorder of fat metabolism (obesity and metabolic syndrome);
- diabetes;
- obstructive sleep apnea syndrome.
What's bothering you?
Diagnostics polycystic ovarian syndrome
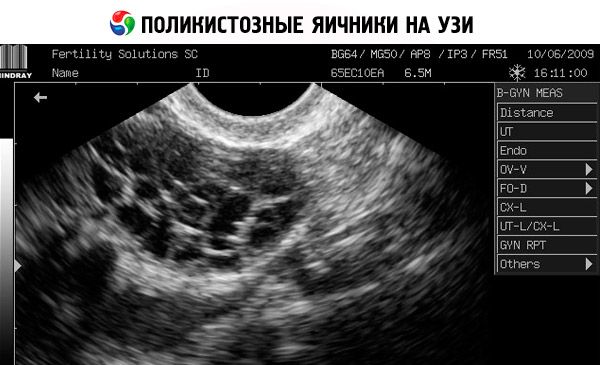
Diagnosis is based on excluding pregnancy (pregnancy test) and examining estradiol, FSH, TSH and prolactin in the blood serum. The diagnosis is confirmed by ultrasonography, which reveals more than 10 follicles in the ovary; the follicles are usually found on the periphery and resemble a string of pearls. If follicles in the ovaries and hirsutism are noted, then testosterone and DHEAS levels should be determined. Pathological levels are assessed as in amenorrhea.
 [ 23 ], [ 24 ], [ 25 ], [ 26 ], [ 27 ], [ 28 ]
[ 23 ], [ 24 ], [ 25 ], [ 26 ], [ 27 ], [ 28 ]
History and physical examination
By carefully collecting anamnesis, hereditary factors for the development of polycystic ovary syndrome are identified. During examination, the body mass index and the waist-to-hip ratio (normally ≤ 0.8) are calculated to diagnose excess body weight and obesity.
Polycystic ovary syndrome is characterized by polymorphism of clinical and laboratory signs.
Special diagnostic methods for polycystic ovary syndrome
A hormonal study is mandatory on the 3rd–5th day of the menstrual-like reaction: the blood levels of LH, FSH, prolactin, testosterone, adrenal androgens - DHEAS, 17-hydroxyprogesterone are determined. Polycystic ovary syndrome is characterized by a high LH/FSH index -> 2.5–3 (due to an increase in LH levels) and hyperandrogenism.
In order to clarify the source of hyperandrogenism, an ACTH test is performed for differential diagnostics with hyperandrogenism caused by a mutation of the gene encoding the 21-hydroxylase enzyme in the adrenal glands (diagnosis of latent and latent forms of adrenogenital syndrome). Technique: at 9 a.m., blood is taken from the cubital vein, then 1 mg of the drug synacthen-depot is administered intramuscularly, and after 9 hours, blood is taken again. The concentration of cortisol and 17-hydroxyprogesterone is determined in both portions of blood, then a coefficient is calculated using a special formula, the values of which should not exceed 0.069. In these cases, the test is negative and the woman (or man) is not a carrier of the 21-hydroxylase gene mutation.
The diphenin test is performed to detect central forms of polycystic ovary syndrome and the possibility of treatment with neurotransmitter drugs. Test technique: the initial concentration of LH and testosterone is determined in the blood, then diphenin is taken 1 tablet 3 times a day for 3 days, after which the concentration of these same hormones is determined in the blood again. The test is considered positive if the level of LH and testosterone decreases.
- Ultrasound of the genitals reveals enlarged ovaries (10 cm3 or more), multiple follicles up to 9 mm in diameter, thickening of the ovarian stroma, and thickening of the capsule.
- Additionally, if insulin resistance is suspected, a glucose tolerance test is performed to determine insulin and glucose levels before and after exercise.
- If adrenal genesis of polycystic ovary syndrome is suspected, genetic counseling and HLA genotyping are recommended.
- Hysterosalpingography.
- Laparoscopy.
- Evaluation of the fertility of the spouse's sperm.
In November 2015, the American Association of Clinical Endocrinologists (AACE), the American College of Endocrinology (ACE), and the Androgen Excess and PCOS Society (AES) released new guidelines for the diagnosis of PCOS. These guidelines are:
- Diagnostic criteria for PCOS must include one of the following three criteria: chronic anovulation, clinical hyperandrogenism, and polycystic ovary disease.
- In addition to clinical findings, serum 17-hydroxyprogesterone and anti-Müllerian hormone levels should be measured to diagnose PCOS.
- Free testosterone levels are more sensitive in detecting androgen excess than total testosterone levels.
What do need to examine?
Who to contact?
Treatment polycystic ovarian syndrome
Women with anovulatory menstrual cycles (history of absent or irregular menses and no evidence of progesterone production), no hirsutism, and no desire to become pregnant are given an intermittent progestin (eg, medroxyprogesterone 5 to 10 mg orally once daily for 10 to 14 days each month for 12 months) or oral contraceptives to reduce the risk of endometrial hyperplasia and cancer and to decrease circulating androgen levels.
Women with polycystic ovary syndrome with anovulatory cycles, with hirsutism and not planning pregnancy, treatment is aimed at reducing hirsutism and regulating serum testosterone and DHEAS levels. Women who want to become pregnant undergo infertility treatment.
Treatment of infertility in polycystic ovary syndrome is carried out in 2 stages:
- Stage 1 - preparatory;
- Stage 2 – ovulation stimulation.
Therapy at the preparatory stage depends on the clinical and pathogenetic form of polycystic ovary syndrome.
- In cases of polycystic ovary syndrome and obesity, it is recommended to prescribe medications that help reduce insulin resistance: the drug of choice is metformin, taken orally at 500 mg 3 times a day for 3–6 months.
- In the ovarian form of polycystic ovary syndrome and high LH levels, drugs are used that help reduce the sensitivity of the hypothalamic-pituitary system to complete suppression of ovarian function (serum estradiol level < 70 pmol/l):
- buserelin spray, 150 mcg in each nostril 3 times a day from the 21st or 2nd day of the menstrual cycle, course 1–3 months, or
- buserelin depot intramuscularly 3.75 mg once every 28 days from the 21st or 2nd day of the menstrual cycle, course 1–3 months, or
- leuprorelin subcutaneously 3.75 mg once every 28 days from the 21st or 2nd day of the menstrual cycle, course 1–3 months, or
- triptorelin subcutaneously 3.75 mg once every 28 days or 0.1 mg once a day from the 21st or 2nd day of the menstrual cycle, course 1–3 months.
It does not matter in principle from which day (21st or 2nd) of the menstrual cycle GnRH agonists are prescribed, however, prescription from the 21st day is preferable, since in this case ovarian cysts do not form. When prescribed from the 2nd day of the cycle, the activation phase preceding the suppression phase in the mechanism of action of the GnRH agonist coincides with the follicular phase of the cycle and can cause the formation of ovarian cysts.
Alternative drugs:
- ethinyl estradiol/dienogest orally 30 mcg/2 mg once a day from the 5th to the 25th day of the menstrual cycle, course 3–6 months or
- ethinyl estradiol/cyproterone acetate orally 35 mcg/2 mg once a day from the 5th to the 25th day of the menstrual cycle, course 3–6 months.
- In the adrenal form of polycystic ovary syndrome, the administration of glucocorticoid drugs is indicated:
- dexamethasone orally 0.25–1 mg once a day, course 3–6 months, or
- methylprednisolone orally 2–8 mg once a day, course 3–6 months, or
- prednisolone orally 2.5–10 mg once a day, course 3–6 months.
- For the central form of polycystic ovary syndrome, anticonvulsants are used:
- diphenin 1 tablet orally 1-2 times a day;
- carbamazepine orally 100 mg 2 times a day, course 3-6 months.
At the 2nd stage, ovulation stimulation is performed.
The choice of drugs and their administration schemes are determined taking into account clinical and laboratory data. During ovulation induction, careful ultrasound and hormonal monitoring of the stimulated cycle is performed.
It is unacceptable to induce ovulation with any medications without ultrasound monitoring. It is inappropriate to start ovulation induction in the presence of cystic formations in the ovaries with a diameter of > 15 mm and an endometrial thickness of > 5 mm.
Ovulation induction with clomiphene is indicated in young women with a short history of the disease and sufficient estrogen levels (serum estradiol < 150 pmol/l) and low LH levels (> 15 IU/l).
Clomiphene is prescribed orally 100 mg once a day from the 5th to the 9th day of the menstrual cycle at the same time of day.
A control ultrasound is performed on the 10th day of the cycle, the diameter of the dominant follicle and the thickness of the endometrium are assessed. Examinations are performed every other day, in the periovulatory period - daily. It is not the day of the cycle that matters, but the size of the leading follicle: if its diameter is more than 16 mm, then it is necessary to perform an ultrasound daily until it reaches a size of 20 mm.
Alternative treatment regimens (with pronounced antiestrogenic effect):
Scheme 1:
- clomiphene orally 100 mg 1 time per day from the 5th to the 9th day of the menstrual cycle at the same time of day +
- ethinyl estradiol (EE) orally 50 mcg 2 times a day from the 10th to the 15th day of the menstrual cycle or
- estradiol orally 2 mg 2 times a day from the 10th to the 15th day of the menstrual cycle.
Scheme 2:
- clomiphene orally 100 mg 1 time per day from the 3rd to the 7th day of the menstrual cycle at the same time of day +
- menotropins intramuscularly 75–150 IU once a day at the same time from the 7th–8th day of the menstrual cycle or
- follitropin alpha intramuscularly 75–150 IU once a day at the same time starting from the 7th–8th day of the menstrual cycle.
Ovulation induction with clomiphene citrate is not indicated in the following situations:
- in hypoestrogenism (serum estradiol level < 150 pmol/l);
- after preliminary preparation with GnRH agonists (as a result of decreased sensitivity of the hypothalamic-pituitary-ovarian system, hypoestrogenism develops);
- in women of advanced reproductive age, with a long history of the disease and high serum LH levels (> 15 IU/L). It is inappropriate to increase the clomiphene dose to 150 mg/day during repeated stimulation courses, as the negative peripheral antiestrogenic effect increases.
It is not recommended to carry out more than 3 consecutive courses of clomiphene stimulation; if the treatment is ineffective, gonadotropins should be used.
Stimulation of ovulation with gonadotropins is indicated in the absence of adequate folliculogenesis after stimulation with clomiphene, in the presence of a pronounced peripheral antiestrogenic effect, insufficient estrogenic saturation. It can be performed both in young patients and in late reproductive age.
Drugs of choice:
- menotropins intramuscularly 150–225 IU once a day from the 3rd–5th day of the menstrual cycle at the same time, course 7–15 days or
- urofollitropin intramuscularly 150–225 IU once a day from the 3rd–5th day of the menstrual cycle at the same time, course 7–15 days.
Alternative drugs (for high risk of developing ovarian hyperstimulation syndrome):
- follitropin alpha intramuscularly 100–150 IU once a day from the 3rd–5th day of the menstrual cycle at the same time, course 7–15 days. Induction of ovulation with gonadotropins using GnRH analogues is indicated in the presence of polycystic ovary syndrome with high levels of LH in the blood serum (> 15 IU/L).
Drugs of choice:
- buserelin in the form of a spray of 150 mcg in each nostril 3 times a day from the 21st day of the menstrual cycle or
- buserelin depot intramuscularly 3.75 mg once on the 21st day of the menstrual cycle;
- leuprorelin subcutaneously 3.75 mg once on the 21st day of the menstrual cycle;
- triptorelin subcutaneously 3.75 mg once on the 21st day of the menstrual cycle or 0.1 mg once a day from the 21st day of the menstrual cycle +
- menotropins intramuscularly 225–300 IU once a day from the 2nd–3rd day of the subsequent menstrual cycle at the same time.
Alternative drugs (for high risk of developing ovarian hyperstimulation syndrome):
- menotropins intramuscularly 150–225 IU once a day from the 2nd–3rd day of the menstrual cycle at the same time or
- follitropin alpha intramuscularly 150–225 IU once a day from the 2nd–3rd day of the menstrual cycle at the same time +
- ganirelix subcutaneously 0.25 mg once a day, starting from the 5th–7th day of gonadotropin use (when the dominant follicle reaches a size of 13–14 mm);
- cetrorelix subcutaneously 0.25 mg once a day, starting from the 5th–7th day of gonadotropin use (when the dominant follicle reaches a size of 13–14 mm).
Induction of ovulation in patients of late reproductive age (with weak ovarian response to gonadotropic drugs).
Drugs of choice:
- menotropins intramuscularly 225 IU once a day from the 3rd to 5th day of the menstrual cycle at the same time +
- triptorelin subcutaneously 0.1 mg once a day from the 2nd day of the menstrual cycle.
Alternative drugs:
- triptorelin subcutaneously 0.1 mg once a day from the 2nd day of the menstrual cycle +
- follitropin alpha intramuscularly 200–225 IU once a day from the 3rd to 5th day of the menstrual cycle at the same time.
In all schemes using gonadotropins, the adequacy of the dose of the latter is assessed by the dynamics of follicle growth (normally 2 mm/day). With slow growth of follicles, the dose is increased by 75 IU, with too rapid growth, it is reduced by 75 IU.
In all schemes, if there is a mature follicle measuring 18–20 mm and the endometrial thickness is at least 8 mm, therapy is stopped and human chorionic gonadotropin is administered intramuscularly at a single dose of 10,000 IU.
After ovulation has been confirmed, the luteal phase of the cycle is supported.
Drugs of choice:
- dydrogesterone orally 10 mg 1-3 times a day, course 10-12 days or
- progesterone orally 100 mg 2-3 times a day, or vaginally 100 mg 2-3 times a day, or intramuscularly 250 mg 1 time per day, course 10-12 days. Alternative drug (in the absence of symptoms of ovarian hyperstimulation):
- human chorionic gonadotropin intramuscularly 1500–2500 IU once a day on days 3.5 and 7 of the luteal phase.
Other medications used in the treatment of PCOS:
- Antiandrogens (eg, spironolactone, leuprolide, finasteride).
- Hypoglycemic drugs (eg, metformin, insulin).
- Selective estrogen receptor modulators (eg, clomiphene citrate).
- Acne medications (eg, benzoyl peroxide, tretinoin cream (0.02-0.1%)/gel (0.01-0.1%)/solution (0.05%), adapalene cream (0.1%)/gel (0.1%, 0.3%)/solution (0.1%), erythromycin 2%, clindamycin 1%, sodium sulfetamide 10%).
Side effects of treatment
When using clomiphene, most patients develop a peripheral antiestrogenic effect, which consists in lagging endometrial growth behind follicle growth and a decrease in the amount of cervical mucus. When using gonadotropins, especially human menopausal gonadotropin (menotropins), ovarian hyperstimulation syndrome (OHSS) may develop; when using recombinant FSH (follitropin alpha), the risk of ovarian hyperstimulation syndrome is lower. When using regimens that include GnRH agonists (triptorelin, buserelin, leuprorelin), the risk of ovarian hyperstimulation syndrome increases, and the use of GnRH agonists can cause symptoms of estrogen deficiency - hot flashes, dry skin and mucous membranes.
Forecast
The effectiveness of infertility treatment in polycystic ovary syndrome depends on the clinical and hormonal characteristics of the course of the disease, the woman's age, the adequacy of preparatory therapy, and the correct selection of the ovulation induction regimen.
In 30% of young women with a short history of the disease, pregnancy can be achieved after preparatory treatment without ovulation induction.
The effectiveness of ovulation stimulation with clomiphene does not exceed 30% per woman; 40% of patients with polycystic ovary syndrome are clomiphene-resistant.
The use of menotropins and urofollitropin allows achieving pregnancy in 45–50% of women, but these drugs increase the risk of developing ovarian hyperstimulation syndrome.
The most effective are the schemes using GnRH agonists, which allow avoiding "parasitic" LH peaks: up to 60% of pregnancies per woman. However, when using these drugs, the highest risk of complications is noted - severe forms of ovarian hyperstimulation syndrome, multiple pregnancy. The use of GnRH antagonists is no less effective, but is not associated with a high risk of ovarian hyperstimulation syndrome.
 [ 34 ]
[ 34 ]

