Medical expert of the article
New publications
Ovary
Last reviewed: 04.07.2025

All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.
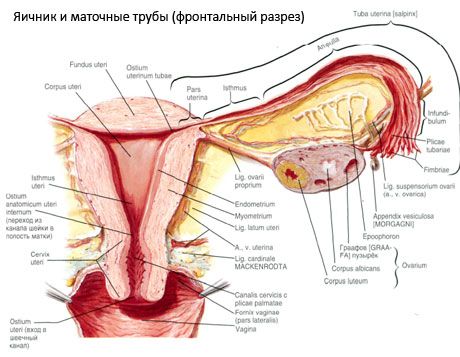
The ovary (ovarium; Greek oophoron) is a paired organ, a female reproductive gland, located in the pelvic cavity behind the broad ligament of the uterus. In the ovaries, female reproductive cells (eggs) develop and mature, and female sex hormones are formed that enter the blood and lymph. The ovary has an ovoid shape, somewhat flattened in the anteroposterior direction. The color of the ovary is pinkish. On the surface of the ovary of a woman who has given birth, depressions and scars are visible - traces of ovulation and the transformation of the corpora lutea. The ovary weighs 5-8 g. The dimensions of the ovary are: length 2.5-5.5 cm, width 1.5-3.0 cm, thickness - up to 2 cm. The ovary has two free surfaces: the medial surface (facies medialis), facing the pelvic cavity, partially covered by the fallopian tube, and the lateral surface (facies lateralis), adjacent to the side wall of the pelvis, to a slightly expressed depression - the ovarian fossa. This fossa is located in the angle between the external iliac vessels covered with peritoneum at the top and the uterine and obturator arteries at the bottom. Behind the ovary, the ureter of the corresponding side passes retroperitoneally from top to bottom.
The surfaces of the ovary pass into a convex free (posterior) edge (margo liber), in front - into the mesenteric edge (margo mesovaricus), attached by means of a short fold of the peritoneum (mesentery of the ovary) to the posterior leaflet of the broad ligament of the uterus. On this anterior edge of the organ there is a grooved depression - the hilum of the ovary (hilum ovarii), through which the artery and nerves enter the ovary, and the veins and lymphatic vessels exit. The ovary also has two ends: the rounded upper tubular end (extremitas tubaria), facing the fallopian tube, and the lower uterine end (extremitas utenna), connected to the uterus by the ovarian ligament (lig. ovarii proprium). This ligament, in the form of a round cord about 6 mm thick, goes from the uterine end of the ovary to the lateral angle of the uterus, located between the two leaves of the broad ligament of the uterus. The ligamentous apparatus of the ovary also includes the ligament suspensory of the ovary (lig.suspensorium ovarii), which is a fold of the peritoneum that runs from the wall of the small pelvis to the ovary and contains the ovarian vessels and bundles of fibrous fibers inside. The ovary is fixed by a short mesentery (mesovarium), which is a duplication of the peritoneum that runs from the posterior leaflet of the broad ligament of the uterus to the mesenteric edge of the ovary. The ovaries themselves are not covered by the peritoneum. The largest ovarian fringe of the fallopian tube is attached to the tubular end of the ovary. The topography of the ovary depends on the position of the uterus, its size (during pregnancy). The ovaries are very mobile organs of the small pelvic cavity.
Vessels and nerves of the ovary
The blood supply to the ovaries is provided by aa. et vv. ovaricae et uterinae. Both ovarian arteries (aa. ovaricae dextra et sinistra) originate from the anterior surface of the aorta just below the renal arteries; the right one usually originates from the aorta, and the left one from the renal artery. Directing downwards and laterally along the anterior surface of the psoas major muscle, each ovarian artery crosses the ureter in front (giving off branches to it), the external iliac vessels, the border line and enters the pelvic cavity, being located here in the suspensory ligament of the ovary. Following in the medial direction, the ovarian artery passes between the leaves of the broad ligament of the uterus under the fallopian tube, giving off branches to it, and then into the mesentery of the ovary; it enters the hilum of the ovary.
The branches of the ovarian artery widely anastomose with the ovarian branches of the uterine artery. Venous outflow from the ovaries is carried out primarily into the ovarian venous plexus, located in the region of the ovarian hilum. From here, the outflow of blood passes in two directions: through the uterine and ovarian veins. The right ovarian vein has valves and flows into the inferior vena cava. The left ovarian vein flows into the left renal vein, although it has no valves.
Lymphatic drainage from the ovaries occurs through the lymphatic vessels, especially abundantly in the area of the organ gate, where the subovarian lymphatic plexus is distinguished. Then the lymph is discharged along the ovarian lymphatic vessels to the paraaortic lymph nodes.
Innervation of the ovaries
Sympathetic - provided by postganglionic fibers from the celiac (solar), superior mesenteric and hypogastric plexuses; parasympathetic - by the visceral sacral nerves.
Structure of the ovary
The surface of the ovary is covered with a single-layer germinal epithelium. Underneath it lies a dense connective tissue protein coat (tunica albuginea). The connective tissue of the ovary forms its stroma (stroma ovarii), rich in elastic fibers. The substance of the ovary, its parenchyma, is divided into outer and inner layers. The inner layer, located in the center of the ovary, closer to its gate, is called the medulla (medulla ovarii). In this layer, in loose connective tissue, there are numerous blood and lymphatic vessels and nerves. The outer layer of the ovary - the cortex (cortex ovarii) is denser. It contains a lot of connective tissue, in which are located the maturing primary ovarian follicles (folliculi ovarici primarii), secondary (vesicular) follicles (folliculi ovarici secundarii, s.vesiculosi), as well as mature follicles, Graafian follicles (folliculi ovarici maturis), as well as yellow and atretic bodies.
Each follicle contains a female reproductive ovum, or oocyte (ovocytus). The ovum is up to 150 µm in diameter, round, contains a nucleus, a large amount of cytoplasm, which, in addition to cellular organelles, contains protein-lipid inclusions (yolk), glycogen, necessary for the nutrition of the ovum. The ovum usually uses up its supply of nutrients within 12-24 hours after ovulation. If fertilization does not occur, the ovum dies.
The human egg has two membranes covering it. Inside is the cytolemma, which is the cytoplasmic membrane of the egg. Outside of the cytolemma is a layer of so-called follicular cells, which protect the egg and perform a hormone-forming function - they secrete estrogens.
The physiological position of the uterus, tubes and ovaries is provided by the suspending, fixing and supporting apparatuses that unite the peritoneum, ligaments and pelvic tissue. The suspending apparatus is represented by paired formations, it includes the round and broad ligaments of the uterus, proper ligaments and suspending ligaments of the ovaries. The broad ligaments of the uterus, proper and suspending ligaments of the ovaries hold the uterus in the middle position. The round ligaments pull the fundus of the uterus forward and provide its physiological tilt.
The fixing (anchoring) apparatus ensures the position of the uterus in the center of the small pelvis and makes it practically impossible for it to shift to the sides, back and forth. But since the ligamentous apparatus departs from the uterus in its lower section, tilts of the uterus in various directions are possible. The fixing apparatus includes ligaments located in the loose tissue of the pelvis and extending from the lower section of the uterus to the lateral, anterior and posterior walls of the pelvis: sacro-magic, cardinal, uterovesical and vesicopubic ligaments.
In addition to the mesovarium, the following ovarian ligaments are distinguished:
- the suspensory ligament of the ovary, previously designated as the infundibulopelvic ligament. It is a fold of the peritoneum with blood vessels (a. et v. ovarica) and lymphatic vessels and nerves of the ovary passing through it, stretched between the lateral wall of the pelvis, the lumbar fascia (in the area of division of the common iliac artery into external and internal) and the upper (tubal) end of the ovary;
- the proper ligament of the ovary passes between the leaves of the broad uterine ligament, closer to the posterior leaf, and connects the lower end of the ovary with the lateral edge of the uterus. The proper ligament of the ovary is attached to the uterus between the beginning of the fallopian tube and the round ligament, behind and above the latter. In the thickness of the ligament pass rr. ovarii, which are the terminal branches of the uterine artery;
- appendicular-ovarian ligament The ligament extends from the mesentery of the appendix to the right ovary or broad ligament of the uterus in the form of a fold of the peritoneum. The ligament is inconstant and is observed in 1/2 - 1/3 of women.
The supporting apparatus is represented by the muscles and fascia of the pelvic floor, divided into lower, middle and upper (inner) layers.
The most powerful is the upper (inner) muscle layer, represented by the paired muscle that lifts the anus. It consists of muscle bundles that fan out from the coccyx to the pelvic bones in three directions (pubococcygeus, iliococcygeus, and ischiococcygeus). This layer of muscles is also called the pelvic diaphragm.
The middle layer of muscles is located between the symphysis, pubic and ischial bones. The middle layer of muscles - the urogenital diaphragm - occupies the anterior half of the pelvic outlet, through which the urethra and vagina pass. In the anterior section between its sheets are muscle bundles that form the external sphincter of the urethra, in the posterior section are muscle bundles that go in the transverse direction - the deep transverse muscle of the perineum.
The lower (outer) layer of the pelvic floor muscles consists of superficial muscles, the shape of which resembles the number 8. These include the bulbocavernous, ischiocavernous, external anal sphincter, and superficial transverse perineal muscle.
Ontogenesis of the ovaries
The process of follicle growth and atresia begins at 20 weeks of pregnancy, and by the time of delivery, up to 2 million oocytes remain in the girl's ovaries. By menarche, their number decreases to 300 thousand. During the entire period of reproductive life, no more than 500 follicles reach maturity and ovulate. The initial growth of follicles does not depend on FSH stimulation, is limited, and atresia quickly occurs. It is believed that instead of steroid hormones, local autocrine/paracrine peptides are the main regulator of growth and atresia of primary follicles. It is believed that the process of follicle growth and atresia is not interrupted by any physiological processes. This process continues at all ages, including the intrauterine period and menopause, and is interrupted by pregnancy, ovulation, and anovulation. The mechanism that triggers follicle growth and their number in each specific cycle is still unclear.
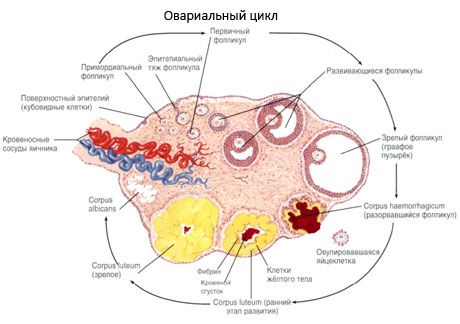
During its development, the follicle undergoes several stages of development. Primordial germ cells originate from the endoderm of the yolk sac, allantois and migrate to the genital area of the embryo at 5-6 weeks of pregnancy. As a result of rapid mitotic division, which continues from 6-8 weeks to 16-20 weeks of pregnancy, up to 6-7 million oocytes are formed in the ovaries of the embryo, surrounded by a thin layer of granulosa cells.
Preantral follicle - the oocyte is surrounded by a membrane (Zona pellucida). Granulosa cells surrounding the oocyte begin to proliferate, their growth depends on gonadotropins and correlates with the level of estrogens. Granulosa cells are the target for FSH. At the preantral follicle stage, granulosa cells are capable of synthesizing three classes of steroids: predominantly induces the activity of aromatase, the main enzyme that converts androgens into estradiol. It is believed that estradiol is capable of increasing the number of its own receptors, exerting a direct mitogenic effect on granulosa cells independent of FSH. It is considered a paracrine factor that enhances the effects of FSH, including the activation of aromatization processes.
FSH receptors appear on the membranes of granulosa cells as soon as follicle growth begins. A decrease or increase in FSH leads to a change in the number of its receptors. This action of FSH is modulated by growth factors. FSH acts through the G-protein, the adenylate cyclase system, although steroidogenesis in the follicle is mainly regulated by FSH, many factors are involved in this process: ion channels, tyrosine kinase receptors, the phospholipase system of second messengers.
The role of androgens in early follicle development is complex. Granulosa cells have androgen receptors. These are not only a substrate for FSH-induced aromatization to estrogens, but can also enhance aromatization at low concentrations. When androgen levels increase, preantral granulosa cells preferentially choose not the aromatization pathway to estrogens but the simpler androgen pathway via 5a-reductase to convert to an androgen that cannot be converted to estrogen, thereby inhibiting aromatase activity. This process also inhibits FSH and LH receptor formation, thus stopping follicle development.
The aromatization process, the follicle with high androgen levels undergoes atresia processes. The growth and development of the follicle depends on its ability to convert androgens into estrogens.
In the presence of FSH, the dominant substance of the follicular fluid will be estrogens. In the absence of FSH - androgens. LH is normally absent in the follicular fluid until the middle of the cycle. As soon as the LH level increases, the mitotic activity of granulosa cells decreases, degenerative changes appear and the androgen level in the follicle increases. The level of steroids in the follicular fluid is higher than in plasma and reflects the functional activity of ovarian cells: granulosa and theca cells. If the only target for FSH is granulosa cells, then LH has many targets - theca cells, stromal and lutein cells and granulosa cells. Both granulosa and theca cells have the ability to steroidogenesis, but aromatase activity predominates in granulosa cells.
In response to LH, theca cells produce androgens, which are then converted by granulosa cells into estrogens through FSH-induced aromatization.
As the follicle grows, theca cells begin to express genes for LH receptors, P450 sec and 3beta-hydroxysteroid dehydrogenase, insulin-like growth factor (IGF-1) synergistically with LH increases gene expression, but does not stimulate steroidogenesis.
Ovarian steroidogenesis is always LH-dependent. As the follicle grows, theca cells express the P450c17 enzyme, which forms androgen from cholesterol. Granulosa cells do not have this enzyme and are dependent on theca cells to produce estrogens from androgens. Unlike steroidogenesis, folliculogenesis is FSH-dependent. As the follicle grows and estrogen levels increase, a feedback mechanism is activated - FSH production is inhibited, which in turn leads to a decrease in the aromatase activity of the follicle and, ultimately, to follicular atresia through apoptosis (programmed cell death).
The feedback mechanism of estrogens and FSH inhibits the development of follicles that have begun to grow, but not the dominant follicle. The dominant follicle contains more FSH receptors, which support the proliferation of granulosa cells and the aromatization of androgens to estrogens. In addition, the paracrine and autocrine pathways act as an important coordinator of antral follicle development.
The autocrine/paracrine regulator consists of peptides (inhibin, activin, follistatin), which are synthesized by granulosa cells in response to FSH and enter the follicular fluid. Inhibin reduces FSH secretion; activin stimulates the release of FSH from the pituitary gland and enhances the action of FSH in the ovary; follistatin suppresses FSH activity, possibly by binding activin. After ovulation and development of the corpus luteum, inhibin is under the control of LH.
The growth and differentiation of ovarian cells is influenced by insulin-like growth factors (IGE). IGF-1 acts on granulosa cells, causing an increase in cyclic adenosine monophosphate (cAMP), progesterone, oxytocin, proteoglycan, and inhibin.
IGF-1 acts on theca cells, causing increased androgen production. Theca cells, in turn, produce tumor necrosis factor (TNF) and epidermal growth factor (EGF), which are also regulated by FSH.
EGF stimulates the proliferation of granulosa cells. IGF-2 is the main growth factor in follicular fluid, and IGF-1, TNF-a, TNF-3, and EGF are also found in it.
Disruption of paracrine and/or autocrine regulation of ovarian function appears to play a role in ovulation disorders and in the development of polycystic ovaries.
As the antral follicle grows, the estrogen content in the follicular fluid increases. At the peak of their increase, receptors for LH appear on the granulosa cells, luteinization of granulosa cells occurs, and progesterone production increases. Thus, in the preovulatory period, an increase in estrogen production causes the appearance of LH receptors, LH, in turn, causes luteinization of granulosa cells and progesterone production. An increase in progesterone reduces estrogen levels, which apparently causes a second peak of FSH in the middle of the cycle.
Ovulation is thought to occur 10-12 hours after the LH peak and 24-36 hours after the estradiol peak. LH is thought to stimulate reduction division of the oocyte, luteinization of granulosa cells, and synthesis of progesterone and prostaglandin in the follicle.
Progesterone enhances the activity of proteolytic enzymes, which together with prostaglandin participate in the rupture of the follicle wall. The progesterone-induced peak of FSH allows the oocyte to exit the follicle by converting plasminogen into the proteolytic enzyme plasmin, and provides a sufficient number of LH receptors for the normal development of the luteal phase.
Within 3 days after ovulation, granulosa cells increase in size, and characteristic vacuoles filled with pigment, lutein, appear in them. Theca-luteal cells differentiate from theca and stroma and become part of the corpus luteum. Capillaries penetrating the corpus luteum develop very quickly under the influence of angiogenesis factors, and with improved vascularization, the production of progesterone and estrogens increases. The activity of steroidogenesis and the lifespan of the corpus luteum are determined by the level of LH. The corpus luteum is not a homogeneous cellular formation. In addition to 2 types of luteal cells, it contains endothelial cells, macrophages, fibroblasts, etc. Large luteal cells produce peptides (relaxin, oxytocin) and are more active in steroidogenesis with greater aromatase activity and greater synthesis of progesterone than small cells.
The peak of progesterone occurs on the 8th day after the LH peak. It is noted that progesterone and estradiol are secreted episodically in the luteal phase in correlation with the pulsatile output of LH. With the formation of the corpus luteum, control of inhibin production passes from FSH to LH. Inhibin increases with the increase in estradiol before the LH peak and continues to increase after the LH peak, although estrogen levels decline. Although inhibin and estradiol are secreted by granulosa cells, they are regulated by different pathways. The decline in inhibin at the end of the luteal phase contributes to the increase in FSH for the next cycle.
The corpus luteum decreases very quickly - on the 9th-11th day after ovulation.
The mechanism of degeneration is unclear and is not related to the luteolytic role of estrogens or to a receptor-related mechanism, as observed in the endometrium. There is another explanation for the role of estrogens produced by the corpus luteum. It is known that estrogens are required for the synthesis of progesterone receptors in the endometrium. Luteal phase estrogens are probably necessary for progesterone-related changes in the endometrium after ovulation. Inadequate development of progesterone receptors, as a consequence of inadequate estrogen levels, is possibly an additional mechanism for infertility and early pregnancy losses, another form of luteal phase deficiency. It is believed that the life span of the corpus luteum is established at the time of ovulation. And it will certainly regress if it is not supported by human chorionic gonadotropin due to pregnancy. Thus, regression of the corpus luteum leads to a decrease in the levels of estradiol, progesterone and inhibin. A decrease in inhibin removes its suppressive effect on FSH; a decrease in estradiol and progesterone allows for a very rapid restoration of GnRH secretion and the removal of the feedback mechanism from the pituitary gland. A decrease in inhibin and estradiol, together with an increase in GnRH, gives rise to FSH over LH. An increase in FSH leads to follicle growth with subsequent selection of a dominant follicle, and a new cycle begins if pregnancy does not occur. Steroid hormones play a leading role in reproductive biology and general physiology. They determine the human phenotype, affect the cardiovascular system, bone metabolism, skin, general well-being and play a key role in pregnancy. The action of steroid hormones reflects the intracellular and genetic mechanisms that are necessary to transmit an extracellular signal to the cell nucleus to cause a physiological response.
Estrogens diffusely penetrate the cell membrane and bind to receptors located in the cell nucleus. The receptor-steroid complex then binds to DNA. In target cells, these interactions lead to gene expression, protein synthesis, and specific cell and tissue function.


 [
[