Medical expert of the article
New publications
Mitral stenosis
Last reviewed: 04.07.2025

All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.
Mitral stenosis is a narrowing of the mitral orifice that prevents blood flow from the left atrium to the left ventricle. The most common cause is rheumatic fever. Symptoms are the same as those of heart failure. The opening tone and diastolic murmur are objectively determined. The diagnosis is established by physical examination and echocardiography. The prognosis is favorable. Drug treatment of mitral stenosis includes diuretics, beta-blockers or heart rate-lowering calcium channel blockers, and anticoagulants. Surgical treatment of more severe cases of mitral stenosis consists of balloon valvulotomy, commissurotomy, or valve replacement.
Epidemiology
Almost always, mitral stenosis is a consequence of acute rheumatic fever. The incidence varies significantly: in developed countries, 1-2 cases per 100,000 population are observed, while in developing countries (for example, in India), rheumatic mitral valve defects are observed in 100-150 cases per 100,000 population.
Causes mitral stenosis
Mitral stenosis is almost always a consequence of acute rheumatic fever (RF). Isolated, "pure" mitral stenosis occurs in 40% of cases among all patients with rheumatic heart disease; in other cases, it is combined with insufficiency and damage to other valves. Rare causes of mitral stenosis include rheumatic diseases (rheumatoid arthritis, systemic lupus erythematosus) and calcification of the mitral ring.
Pathogenesis
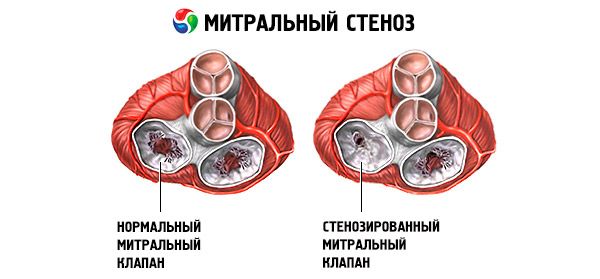
In rheumatic mitral stenosis, there is compaction, fibrosis and calcification of the valve cusps, fusion along the commissures with frequent involvement of the chords. Normally, the area of the mitral orifice is 4-6 cm 2, and the pressure in the left atrium does not exceed 5 mm Hg. When the left atrioventricular orifice narrows to 2.5 cm 2, an obstacle to the normal blood flow from the left atrium to the left ventricle occurs and the valve pressure gradient begins to increase. As a result, the pressure in the left atrium increases to 20-25 mm Hg. The resulting pressure gradient between the left atrium and the left ventricle promotes the movement of blood through the narrowed orifice.
As stenosis progresses, the transmitral pressure gradient increases, which helps maintain diastolic blood flow through the valve. According to Gorlin's formula, the mitral valve area (5MC) is determined by the values of the transmitral gradient (MG) and mitral blood flow (MBF):
BMK - MK/37.7 • ∆DM
The main hemodynamic consequence of mitral valve defects is congestion in the pulmonary circulation (PC). With a moderate increase in pressure in the left atrium (no more than 25-30 mm Hg), blood flow in the PC is impeded. The pressure in the pulmonary veins increases and is transmitted through the capillaries to the pulmonary artery, resulting in the development of venous (or passive) pulmonary hypertension. With an increase in pressure in the left atrium of more than 25-30 mm Hg, the risk of rupture of the pulmonary capillaries and the development of alveolar pulmonary edema increases. To prevent these complications, a protective reflex spasm of the pulmonary arterioles occurs. As a result, the blood flow to the cellular capillaries from the right ventricle decreases, but the pressure in the pulmonary artery increases sharply (arterial, or active, pulmonary hypertension develops).
In the early stages of the disease, the pressure in the pulmonary artery increases only during physical or emotional stress, when the blood flow in the ICC should increase. Late stages of the disease are characterized by high values of pressure in the pulmonary artery even at rest and an even greater increase under stress. Long-term existence of pulmonary hypertension is accompanied by the development of proliferative and sclerotic processes in the wall of the ICC arterioles, which gradually obliterate. Despite the fact that the occurrence of arterial pulmonary hypertension can be considered as a compensatory mechanism, due to the decrease in capillary blood flow, the diffusion capacity of the lungs also drops sharply, especially under stress, i.e. the mechanism of progression of pulmonary hypertension due to hypoxemia is activated. Alveolar hypoxia causes pulmonary vasoconstriction by direct and indirect mechanisms. The direct effect of hypoxia is associated with depolarization of vascular smooth muscle cells (mediated by a change in the function of potassium channels in cell membranes) and their contraction. The indirect mechanism involves the action of endogenous mediators (such as leukotrienes, histamine, serotonin, angiotensin II, and catecholamines) on the vascular wall. Chronic hypoxemia leads to endothelial dysfunction, which is accompanied by a decrease in the production of endogenous relaxing factors, including prostacyclin, prostaglandin E2, and nitric oxide. Long-term endothelial dysfunction leads to obliteration of pulmonary vessels and endothelial damage, which in turn leads to increased blood clotting, proliferation of smooth muscle cells with a tendency to thrombus formation in situ, and an increased risk of thrombotic complications with the development of subsequent chronic postthrombotic pulmonary hypertension.
The causes of pulmonary hypertension in mitral valve defects, including mitral stenosis, are:
- passive transmission of pressure from the left atrium to the pulmonary venous system;
- spasm of the pulmonary arterioles in response to increased pressure in the pulmonary veins;
- swelling of the walls of small pulmonary vessels;
- obliteration of pulmonary vessels with endothelial damage.
The mechanism of mitral stenosis progression remains unclear to this day. A number of authors consider the main factor to be current valvulitis (often subclinical), while others assign the leading role to traumatization of valve structures by turbulent blood flow with thrombotic masses deposited on the valves, which underlies the narrowing of the mitral orifice.
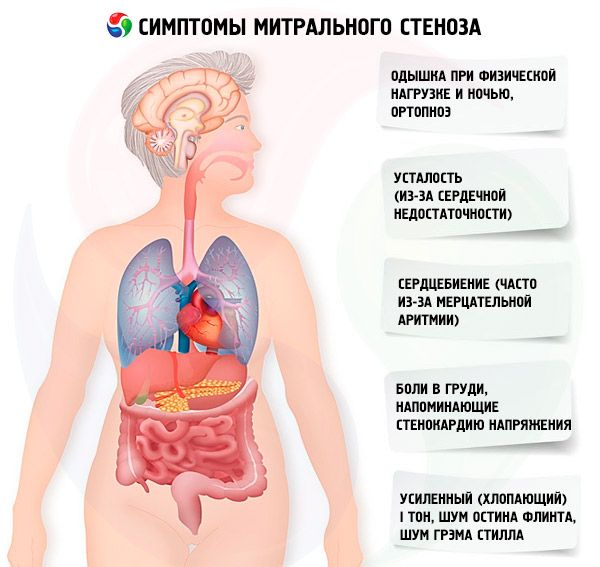
Symptoms mitral stenosis
The symptoms of mitral stenosis correlate poorly with the severity of the disease, since in most cases the pathology progresses slowly, and patients reduce their activity without noticing it. Many patients have no clinical manifestations until pregnancy or the development of atrial fibrillation. Initial symptoms are usually those of heart failure (dyspnea on exertion, orthopnea, paroxysmal nocturnal dyspnea, fatigue). They usually appear 15-40 years after the episode of rheumatic fever, but in developing countries symptoms may appear even in children. Paroxysmal or persistent atrial fibrillation worsens existing diastolic dysfunction, causing pulmonary edema and acute dyspnea if the ventricular rate is poorly controlled.
Atrial fibrillation may also present with palpitations; in 15% of patients not receiving anticoagulants, this causes systemic embolism with limb ischemia or stroke.
Less common symptoms include hemoptysis due to rupture of small pulmonary vessels and pulmonary edema (especially during pregnancy when blood volume increases); dysphonia due to compression of the left recurrent laryngeal nerve by an enlarged left atrium or pulmonary artery (Ortner's syndrome); symptoms of pulmonary arterial hypertension and right ventricular failure.
The first symptoms of mitral stenosis
With a mitral orifice area >1.5 cm2 , symptoms may be absent, but an increase in transmitral blood flow or a decrease in diastolic filling time lead to a sharp increase in pressure in the left atrium and the appearance of symptoms. Trigger factors for decompensation: physical exertion, emotional stress, atrial fibrillation, pregnancy.
The first symptom of mitral stenosis (in approximately 20% of cases) may be an embolic event, most often a stroke with the development of persistent neurological deficit in 30-40% of patients. One third of thromboembolisms develop within 1 month after the development of atrial fibrillation, two thirds - within the first year. The source of embolism is usually thrombi located in the left atrium, especially in its appendage. In addition to strokes, embolisms to the spleen, kidneys, and peripheral arteries are possible.
In sinus rhythm, the risk of embolism is determined by:
- age;
- left atrial thrombosis;
- mitral orifice area;
- concomitant aortic insufficiency.
In the case of permanent atrial fibrillation, the risk of embolism increases significantly, especially if the patient has a history of similar complications. Spontaneous contrast enhancement of the left atrium during transesophageal echocardiography is also considered a risk factor for systemic embolism.
With an increase in pressure in the ICC (especially at the stage of passive pulmonary hypertension), complaints of shortness of breath during physical exertion appear. As stenosis progresses, shortness of breath occurs with lesser loads. It should be remembered that complaints of shortness of breath may be absent even with undoubted pulmonary hypertension, since the patient may lead a sedentary lifestyle or subconsciously limit daily physical activity. Paroxysmal nocturnal dyspnea occurs as a result of blood stagnation in the ICC when the patient is lying down as a manifestation of interstitial pulmonary edema and a sharp increase in blood pressure in the vessels of the ICC. Due to an increase in pressure in the pulmonary capillaries and the exudation of plasma and erythrocytes into the lumen of the alveoli, hemoptysis may develop.
Patients often complain of increased fatigue, palpitations, and irregular heartbeats. Transient hoarseness of the voice (Ortner's syndrome) may be observed. This syndrome occurs as a result of compression of the recurrent nerve by the enlarged left atrium.
Patients with mitral stenosis often experience chest pains resembling angina. The most likely causes are pulmonary hypertension and right ventricular hypertrophy.
In severe decompensation, facies mitralis (a bluish-pink flush on the cheeks that is associated with decreased ejection fraction, systemic vasoconstriction, and right-sided heart failure), epigastric pulsation, and signs of right ventricular heart failure may be observed.
 [ 21 ]
[ 21 ]
Inspection and auscultation
On inspection and palpation, distinct I (S1) and II (S2) heart sounds can be detected. S1 is best palpated at the apex and S2 at the left upper sternal border. The pulmonary component of S3 (P) is responsible for the impulse and is a result of pulmonary arterial hypertension. Visible RV pulsation, palpated at the left sternal border, may accompany jugular venous distension if pulmonary arterial hypertension exists and right ventricular diastolic dysfunction develops.
The apical impulse in mitral stenosis is most often normal or decreased, which reflects the normal function of the left ventricle and a decrease in its volume. Palpable 1st tone in the precordial region indicates preserved mobility of the anterior mitral valve leaflet. In the fawn-sided position, diastolic tremor can be palpated. With the development of pulmonary hypertension, a cardiac impulse is noted along the right border of the sternum.
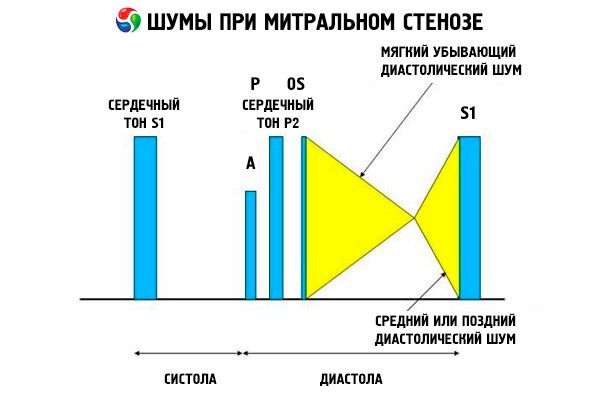
The auscultatory picture of mitral stenosis is quite characteristic and includes the following signs:
- amplified (clapping) 1st tone, the intensity of which decreases as the stenosis progresses;
- the opening tone of the mitral valve following the second tone, which disappears with valve calcification;
- diastolic murmur with a maximum at the apex (mesodiastolic, presystolic, pandiastolic), which must be listened to in the left lateral position.
Auscultation reveals a loud S 1 caused by the cusps of the stenotic mitral valve closing abruptly, like an "inflating" sail; this phenomenon is heard best at the apex. A split S with an enlarged P due to pulmonary arterial hypertension is also commonly heard. The most prominent is the early diastolic snap of the cusps opening into the left ventricle (LV), which is loudest at the left lower sternal border. It is accompanied by a low, crescendo-rumbling diastolic murmur, which is heard best with a funnel-shaped stethoscope at the apex of the heart (or over the palpable apical impulse) at end-expiration when the patient is lying on the left side. The opening sound may be soft or absent if the mitral valve is sclerotic, fibrotic, or thickened. The click moves closer to the P (increasing the duration of the murmur) as the severity of mitral stenosis increases and the left atrial pressure increases. The diastolic murmur increases with the Valsalva maneuver (when blood flows into the left atrium), after exercise, and with squatting and shaking hands. It may be less pronounced if the enlarged right ventricle displaces the left ventricle posteriorly and when other disorders (pulmonary arterial hypertension, right-sided valve disease, atrial fibrillation with a rapid ventricular rate) reduce blood flow through the mitral valve. The presystolic increase is due to narrowing of the mitral valve orifice during left ventricular contraction, which also occurs in atrial fibrillation, but only at the end of the short diastole, when the left atrial pressure is still high.
The following diastolic murmurs may be associated with the murmur of mitral stenosis:
- Graham Still's murmur (a soft, decrescendo diastolic murmur heard best at the left sternal border and caused by pulmonary valve regurgitation due to severe pulmonary hypertension);
- Austin-Flint murmur (a mid- to late-diastolic murmur heard at the apex of the heart and caused by the effect of aortic regurgitant flow on the mitral valve leaflets) when rheumatic carditis affects the mitral and aortic valves.
Disorders that cause diastolic murmurs that mimic the murmur of mitral stenosis include mitral regurgitation (due to large flow through the mitral orifice), aortic regurgitation (causing an Austin-Flint murmur), and atrial myxoma (which causes a murmur that typically changes in loudness and position with each heartbeat).
Mitral stenosis can cause symptoms of pulmonary heart disease. The classic sign of facies mitralis (plum-colored skin flushing in the malar bone area) occurs only when cardiac function is low and pulmonary hypertension is severe. Causes of facies mitralis include dilated skin vessels and chronic hypoxemia.
Sometimes the first symptoms of mitral stenosis are manifestations of embolic stroke or endocarditis. The latter rarely occurs in mitral stenosis not accompanied by mitral regurgitation.
 [ 22 ], [ 23 ], [ 24 ], [ 25 ], [ 26 ], [ 27 ]
[ 22 ], [ 23 ], [ 24 ], [ 25 ], [ 26 ], [ 27 ]
Clinical manifestations of pulmonary hypertension in mitral stenosis
The first symptoms of pulmonary hypertension are nonspecific, which makes its early diagnosis much more difficult.
Dyspnea is caused by both the presence of pulmonary hypertension and the inability of the heart to increase cardiac output during exercise. Dyspnea is usually inspiratory in nature, is inconstant at the onset of the disease, and occurs only during moderate physical exertion, then, as the pressure in the pulmonary artery increases, it appears during minimal physical exertion and may be present at rest. With high pulmonary hypertension, a dry cough may occur. It should be remembered that patients may subconsciously limit physical activity, adapting to a certain lifestyle, so complaints of dyspnea are sometimes absent even with undoubted pulmonary hypertension.
Weakness, increased fatigue - the causes of these complaints may be a fixed cardiac output (the amount of blood ejected into the aorta does not increase in response to physical exertion), increased pulmonary vascular resistance, as well as decreased perfusion of peripheral organs and skeletal muscles due to impaired peripheral circulation.
Dizziness and fainting are caused by hypoxic encephalopathy and are usually provoked by physical exertion.
Persistent pain behind the sternum and to the left of it is caused by overstretching of the pulmonary artery, as well as insufficient blood supply to the hypertrophied myocardium (relative coronary insufficiency).
Heart palpitations and irregular heartbeats. These symptoms are associated with the frequent occurrence of atrial fibrillation.
Hemoptysis occurs as a result of rupture of pulmonary-bronchial anastomoses under the influence of high venous pulmonary hypertension, and can also be a consequence of increased pressure in the pulmonary capillaries and leakage of plasma and erythrocytes into the lumen of the alveoli. Hemoptysis can also be a symptom of pulmonary embolism and pulmonary infarction.
To characterize the severity of pulmonary hypertension, the functional classification proposed by WHO for patients with circulatory failure is used:
- class I - patients with pulmonary hypertension, but without limitation of physical activity. Normal physical activity does not cause shortness of breath, weakness, chest pain, dizziness;
- class II - patients with pulmonary hypertension, leading to some reduction in physical activity. At rest, they feel comfortable, but normal physical activity is accompanied by the appearance of shortness of breath, weakness, chest pain, dizziness;
- class III - patients with pulmonary hypertension, leading to a marked limitation of physical activity. At rest, they feel comfortable, but slight physical activity causes shortness of breath, weakness, chest pain, dizziness;
- class IV - patients with pulmonary hypertension who cannot perform any physical activity without the listed symptoms. Shortness of breath or weakness are sometimes present even at rest, discomfort increases with minimal physical activity.
Where does it hurt?
Forms
Mitral stenosis is classified according to severity (ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography).
Classification of mitral stenosis by degree
Degree of stenosis |
Mitral orifice area, cm2 |
Transmitral gradient, mmHg |
Systolic pressure in the pulmonary artery, mm Hg |
Easy |
>1.5 |
<5 |
<30 |
Moderate |
1.0-1.5 |
5-10 |
30-50 |
Heavy |
<1 0 |
>10 |
>50 |
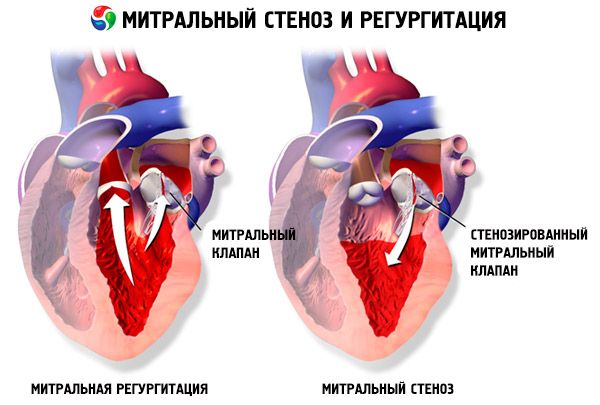
In mitral stenosis, the mitral valve leaflets become thickened and immobile, and the mitral orifice narrows due to fusion of the commissures. The most common cause is rheumatic fever, although most patients do not recall having the disease. Rarer causes include congenital mitral stenosis, infective endocarditis, systemic lupus erythematosus, atrial myxoma, rheumatoid arthritis, and malignant carcinoid syndrome with right-to-left atrial shunting. Mitral regurgitation (MR) may coexist with mitral stenosis if the valve fails to close completely. Many patients with mitral stenosis due to rheumatic fever also have aortic regurgitation.
The normal mitral valve orifice area is 4–6 cm 2. An area of 1–2 cm 2 indicates moderate to severe mitral stenosis and often causes clinical symptoms during exercise. An area < 1 cm 2 represents critical stenosis and may cause symptoms at rest. Left atrial size and pressure progressively increase to compensate for mitral stenosis. Pulmonary venous and capillary pressures also increase and may cause secondary pulmonary hypertension, leading to right ventricular failure and tricuspid and pulmonary regurgitation. The rate of progression of the pathology varies.
Pathology of the valves with dilation of the left atrium predisposes to the development of atrial fibrillation (AF) and thromboembolism.
Diagnostics mitral stenosis
The preliminary diagnosis is made clinically and confirmed by echocardiography. Two-dimensional echocardiography provides information on the degree of valvular calcification, left atrial size, and stenosis. Doppler echocardiography provides information on the transvalvular gradient and pulmonary artery pressure. Transesophageal echocardiography can be used to detect or exclude small thrombi in the left atrium, especially in the left atrial appendage, which are often undetectable by transthoracic examination.
Chest radiography typically shows effacement of the left cardiac border due to a dilated left atrial appendage. The main trunk of the pulmonary artery may be visible; the diameter of the descending right pulmonary artery exceeds 16 mm if pulmonary hypertension is severe. The pulmonary veins of the upper lobes may be dilated because the veins of the lower lobes are compressed, causing upper lobe congestion. A double shadow of an enlarged left atrium may be seen along the right cardiac outline. Horizontal lines in the lower posterior lung fields (Kerley lines) indicate interstitial edema associated with high left atrial pressure.
Cardiac catheterization is prescribed only for preoperative detection of coronary heart disease: it is possible to assess the enlargement of the left atrium, the pressure in the pulmonary arteries and the valve area.
The patient's ECG is characterized by the appearance of a P-mitral (wide, with a PQ notch), a deviation of the electrical axis of the heart to the right, especially with the development of pulmonary hypertension, as well as hypertrophy of the right (with isolated mitral stenosis) and left (with combination with mitral insufficiency) ventricles.
The severity of stenosis is assessed using Doppler ultrasound. The mean transmitral pressure gradient and the area of the mitral valve can be determined quite accurately using continuous wave technology. Of great importance is the assessment of the degree of pulmonary hypertension, as well as concomitant mitral and aortic regurgitation.
Additional information can be obtained using a stress test (stress echocardiography) with recording of transmitral and tricuspid blood flow. If the mitral valve area is < 1.5 cm2 and the pressure gradient is > 50 mmHg (after stress), balloon mitral valvuloplasty should be considered.
In addition, spontaneous echo contrast during transesophageal echocardiography is an independent predictor of embolic complications in patients with mitral stenosis.
Transesophageal echocardiography allows to clarify the presence or absence of a left atrial thrombus, to clarify the degree of mitral regurgitation in planned balloon mitral valvuloplasty. In addition, transesophageal examination allows to accurately assess the state of the valve apparatus and the severity of changes in subvalvular structures, as well as to assess the likelihood of restenosis.
Cardiac and major vessel catheterization is performed when surgical intervention is planned and noninvasive testing data do not provide a definitive result. Direct measurement of left atrial and left ventricular pressure requires transseptal catheterization, which is associated with unjustified risk. An indirect method for measuring left atrial pressure is pulmonary artery wedge pressure.
What do need to examine?
Differential diagnosis
With careful examination, the diagnosis of mitral valve disease is usually beyond doubt.
Mitral stenosis is also differentiated from left atrial myxoma, other valve defects (mitral insufficiency, tricuspid stenosis), atrial septal defect, pulmonary vein stenosis, and congenital mitral stenosis.
 [ 51 ], [ 52 ], [ 53 ], [ 54 ], [ 55 ], [ 56 ], [ 57 ]
[ 51 ], [ 52 ], [ 53 ], [ 54 ], [ 55 ], [ 56 ], [ 57 ]
Examples of diagnosis formulation
- Rheumatic heart disease. Combined mitral valve disease with predominant stenosis of the left atrioventricular orifice grade III. Atrial fibrillation, permanent form, tachysystole. Moderate pulmonary hypertension. NK PB stage, III FC.
- Rheumatic heart disease. Combined mitral valve defect. Mitral valve replacement (Medinzh - 23) from DD/MM/GG. NK stage IIA, II FC.
Who to contact?
Treatment mitral stenosis
The main goals of treatment of patients with mitral stenosis are to improve the prognosis and increase life expectancy, and to alleviate the symptoms of the disease.
Asymptomatic patients are advised to limit intense physical activity. In cases of decompensation and chronic heart failure, sodium restriction in food is recommended.
Drug treatment of mitral stenosis
Drug therapy can be used to control the symptoms of mitral stenosis, for example in preparation for surgery. Diuretics reduce left atrial pressure and relieve symptoms associated with mitral stenosis. However, diuretics should be used with caution because they may reduce cardiac output. Beta-blockers and calcium channel blockers (verapamil and diltiazem) reduce heart rate at rest and during exercise, improving left ventricular filling by prolonging diastole. These drugs can relieve symptoms associated with physical activity and are especially indicated in sinus tachycardia and atrial fibrillation.
Atrial fibrillation is a common complication of mitral stenosis, especially in older people. The risk of thromboembolism in the presence of atrial fibrillation increases significantly (10-year survival is 25% of patients compared to 46% in patients with sinus rhythm).
Indirect anticoagulants (warfarin, starting dose 2.5-5.0 mg, under INR control) are indicated;
- all patients with mitral stenosis complicated by atrial fibrillation (paroxysmal, persistent or permanent form);
- patients with a history of embolic events, even with preserved sinus rhythm;
- patients with a thrombus in the left atrium;
- patients with severe mitral stenosis and those patients whose left atrium size is >55 mm.
Treatment is carried out under the control of INR, the target levels of which are from 2 to 3. If the patient develops embolic complications despite the anticoagulant treatment, it is recommended to add acetylsalicylic acid at a dose of 75-100 mg / day (alternatives are dipyridamole or clopidogrel). It should be noted that randomized controlled trials of the use of anticoagulants in patients with mitral stenosis have not been conducted; recommendations are based on extrapolation of data obtained in cohorts of patients with atrial fibrillation.
Since the development of atrial fibrillation in a patient with mitral stenosis is accompanied by decompensation, treatment aimed at slowing the ventricular rhythm is of primary importance. As already mentioned, beta-blockers, verapamil or diltiazem may be the drugs of choice. Digoxin may also be used, but its narrow therapeutic interval and worse ability to prevent heart rate acceleration during exercise limit its use compared to beta-blockers. Electrical cardioversion is also of limited use in persistent atrial fibrillation, since without surgical treatment of atrial fibrillation the probability of relapse is very high.
Surgical treatment of mitral stenosis
The main method of treating mitral stenosis is surgical, since today there is no drug treatment that can slow the progression of stenosis.
Patients with more severe symptoms or evidence of pulmonary arterial hypertension require valvotomy, commissurotomy, or valve replacement.
The procedure of choice is percutaneous balloon mitral valvuloplasty. This is the main method of surgical treatment of mitral stenosis. In addition, open commissurotomy and mitral valve replacement are used.
Percutaneous balloon valvotomy is the preferred technique for younger patients, older patients who cannot tolerate more invasive procedures, and patients without significant valvular calcification, subvalvular deformity, left atrial thrombi, or significant mitral regurgitation. In this procedure, under echocardiographic guidance, a balloon is passed across the atrial septum from the right to the left atrium and inflated to separate the fused mitral valve leaflets. Outcomes are comparable to those of more invasive procedures. Complications are rare and include mitral regurgitation, embolism, left ventricular perforation, and an atrial septal defect, which is likely to persist if the interatrial pressure difference is large.
Percutaneous balloon mitral valvuloplasty is indicated for the following groups of patients with a mitral orifice area of less than 1.5 cm 2:
- decompensated patients with favorable characteristics for percutaneous mitral valvuloplasty (class I, level of evidence B);
- decompensated patients with contraindications to surgical treatment or high surgical risk (class I, level of evidence! I C);
- in case of planned primary surgical correction of the defect in patients with unsuitable valve morphology, but with satisfying clinical characteristics (class IIa, level of evidence C);
- "asymptomatic" patients with suitable morphological and clinical characteristics, high risk of thromboembolic complications or high risk of decompensation of hemodynamic parameters;
- with a history of embolic complications (class IIa, level of evidence C);
- with the phenomenon of spontaneous echo contrast in the left atrium (class IIa, level of evidence C);
- with permanent or paroxysmal atrial fibrillation (class IIa, level of evidence C);
- with pulmonary artery systolic pressure greater than 50 mmHg (class IIa, level of evidence C);
- when major non-cardiac surgeries are required (class IIa, level of evidence C);
- in case of pregnancy planning (class IIa, level of evidence C).
Suitable characteristics for percutaneous mitral valvuloplasty are the absence of the following features:
- clinical: old age, history of commissurotomy, functional class IV heart failure, atrial fibrillation, severe pulmonary hypertension;
- Morphological: mitral valve calcification of any degree, assessed by fluorography, very small mitral valve area, severe tricuspid regurgitation.
Patients with severe subvalvular disease, valvular calcification, or thrombi in the left atrium may be candidates for commissurotomy, in which the fused mitral valve leaflets are separated using a dilator passed through the left atrium and left ventricle (closed commissurotomy) or manually (open commissurotomy). Both procedures require a thoracotomy. The choice depends on the surgical situation and the degree of fibrosis and calcification.
Mitral valve plastic surgery (open commissurotomy) or replacement is performed for the following class I indications.
In the presence of heart failure III-IV FC and moderate or severe mitral stenosis in cases where:
- mitral balloon valvuloplasty cannot be performed;
- Mitral balloon valvuloplasty is contraindicated due to thrombus in the left atrium despite the use of anticoagulants, or due to concomitant moderate or severe mitral regurgitation;
- valve morphology is not suitable for mitral balloon valvuloplasty.
In moderate to severe mitral stenosis and concomitant moderate to severe mitral regurgitation (valve replacement is indicated if valve repair is not possible).
Valve replacement is a last resort. It is prescribed to patients with a mitral valve area < 1.5 cm 2, moderate to severe symptoms, and valve pathology (eg, fibrosis) that prevents the use of other methods.
Mitral valve replacement is advisable (class IIa indications) in severe mitral stenosis and severe pulmonary hypertension (pulmonary artery systolic pressure over 60 mm Hg), symptoms of class I-II heart failure, unless mitral balloon valvuloplasty or mitral valve replacement is suggested. Patients with mitral stenosis who do not have symptoms of decompensation should be examined annually. The examination includes collection of complaints, anamnesis, examination, chest X-ray and ECG. If the patient's condition has changed over the previous period or the results of the previous examination indicate severe mitral stenosis, echocardiography is indicated. In all other cases, annual echocardiography is not necessary. If the patient complains of palpitations, 24-hour (Holter) ECG monitoring is recommended to detect paroxysms of atrial fibrillation.
During pregnancy, patients with mild to moderate stenosis can receive only drug treatment. The use of diuretics and beta-blockers is safe. If anticoagulant treatment is necessary, patients are prescribed heparin injections, since warfarin is contraindicated.
Prevention
The most important issue of the tactics of further management of patients with mitral stenosis is the prevention of relapses of rheumatic fever with prolonged-release penicillin drugs prescribed for life, as well as to all patients after surgical correction of the defect (including for the prevention of infective endocarditis). Benzathine benzylpenicillin is prescribed at a dose of 2.4 million U for adults and 1.2 million U for children intramuscularly once a month.
All patients with mitral stenosis are indicated for secondary prevention of relapses of rheumatic fever. In addition, all patients are indicated for prevention of infective endocarditis.
Asymptomatic patients require only prophylaxis against recurrent rheumatic fever [eg, intramuscular injections of benzylpenicillin (penicillin G sodium salt sterile) 1.2 million units every 3 or 4 weeks] until age 25–30 years and endocarditis prophylaxis before risky procedures.
Forecast
The natural history of mitral stenosis varies, but the time between the onset of symptoms and severe disability is approximately 7 to 9 years. The outcome of treatment depends on the patient's age, functional status, pulmonary arterial hypertension, and the degree of atrial fibrillation. The results of valvotomy and commissurotomy are equivalent, with both methods restoring valve function in 95% of patients. However, function deteriorates over time in most patients, and many require a repeat procedure. Risk factors for death include atrial fibrillation and pulmonary hypertension. The cause of death is usually heart failure or pulmonary or cerebrovascular embolism.
Mitral stenosis usually progresses slowly and has a long period of compensation. More than 80% of patients survive for 10 years without symptoms or moderate signs of CHF (I-II FC according to NUHA). The 10-year survival rate of decompensated and non-operated patients is significantly worse and does not exceed 15%. With the development of severe pulmonary hypertension, the average survival period does not exceed 3 years.
 [ 74 ]
[ 74 ]

