Medical expert of the article
New publications
Diabetes mellitus in pregnancy
Last reviewed: 04.07.2025

All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.
Diabetes mellitus in pregnancy is a group of metabolic diseases characterized by hyperglycemia that results from defects in insulin secretion, insulin action, or both. Chronic hyperglycemia in diabetes leads to damage and failure of various organs, especially the eyes, kidneys, nervous system, and cardiovascular system.
Gestational diabetes can be classified as A1GDM and A2GDM. Gestational diabetes that is managed without medications and responds to diet therapy is diet-controlled gestational diabetes or A1GDM. On the other hand, gestational diabetes that is managed with medications to achieve adequate glycemic control is A2GDM. [ 1 ], [ 2 ], [ 3 ], [ 4 ], [ 5 ]
Epidemiology
Diabetes mellitus (DM) is a metabolic disorder resulting from impaired insulin production, impaired insulin action, or both. It is a major noncommunicable disease that is increasing worldwide, causing 4.8 million deaths and morbidity in 371 million people annually. In recent years, patterns of change in the age of onset of DM have been observed, with younger populations now disproportionately affected. An estimated 28 million women of reproductive age currently have DM worldwide. Most of these women have type 2 DM, and 80% of this burden occurs in low- and middle-income countries. [ 6 ]
According to various data, from 1 to 14% of all pregnancies (depending on the population studied and the diagnostic methods used) are complicated by gestational diabetes.
The prevalence of diabetes mellitus types 1 and 2 among women of reproductive age is 2%, in 1% of all pregnancies the woman initially has diabetes, in 4.5% of cases gestational diabetes develops, including in 5% of cases diabetes mellitus manifests under the guise of gestational diabetes.
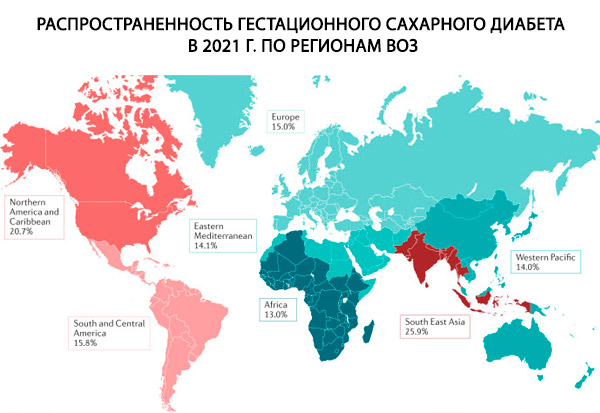
During pregnancy, diabetes mellitus can be either pre-existing (type 1 or 2) or gestational diabetes mellitus (GDM). In pre-existing diabetes, risk factors such as genetic predisposition, family history of type 1 diabetes, and autoimmune disorders play a critical role in the development of type 1 diabetes.[ 7 ] Factors that play a significant role in both type 2 diabetes and GDM include obesity, unhealthy diet, physical inactivity, family history of type 2 diabetes, maternal age, and ethnicity.[ 8 ] Other lifestyle changes such as alcohol abuse and smoking are also associated with the etiology of type 2 diabetes.
The causes of increased fetal morbidity are macrosomia, hypoglycemia, congenital defects, respiratory failure syndrome, hyperbilirubinemia, hypocalcemia, polycythemia, hypomagnesemia. Below is the classification of P. White, which characterizes the numerical (p, %) probability of the birth of a viable child depending on the duration and complications of maternal diabetes.
- Class A. Impaired glucose tolerance and absence of complications - p=100;
- Class B. Duration of diabetes less than 10 years, onset at the age of over 20 years, no vascular complications - p=67;
- Class C. Duration from 10 to 19 years, occurred at 10-19 years, no vascular complications - p=48;
- Class D. Duration more than 20 years, occurred before 10 years; retinopathy or calcification of leg vessels - p=32;
- Class E. Calcification of pelvic vessels - p=13;
- Class F. Nephropathy - p=3.
Causes diabetes mellitus in pregnancy
Gestational diabetes, or gestagenic diabetes (GDM), is a glucose intolerance disorder (GT) that occurs during pregnancy and resolves after delivery. The diagnostic criterion for this type of diabetes is exceeding any two of the following three capillary blood glucose levels, mmol/l: fasting - 4.8, 1 hour after - 9.6, and 2 hours after - 8 after an oral load of 75 g of glucose.
Impaired glucose tolerance during pregnancy reflects the physiological effects of counterinsular placental hormones and insulin resistance and occurs in approximately 2% of pregnant women. Early detection of impaired glucose tolerance is important for two reasons: first, 40% of women with a history of gestational diabetes develop clinical diabetes within 6-8 years and therefore require follow-up; second, impaired glucose tolerance increases the risk of perinatal mortality and fetopathy, as does diabetes mellitus.
The etiology of gestational diabetes appears to be related to
- dysfunction of pancreatic beta cells or delayed beta cell response to glycemic levels and
- severe insulin resistance secondary to the release of placental hormones.
Human placental lactogen is the main hormone associated with increased insulin resistance in GDM. Other hormones associated with the development of this disease include growth hormone, prolactin, corticotropin-releasing hormone, and progesterone; these hormones contribute to the stimulation of insulin resistance and hyperglycemia during pregnancy.
Risk factors
During a pregnant woman's first visit to the doctor, it is necessary to assess her risk of developing gestational diabetes, since further diagnostic tactics depend on it. The low-risk group for developing gestational diabetes includes women under 25 years of age, with normal body weight before pregnancy, no history of diabetes in first-degree relatives, no history of carbohydrate metabolism disorders (including glucosuria), and an uncomplicated obstetric history. To classify a woman as a low-risk group for developing gestational diabetes, all of the listed signs must be present. In this group of women, testing using load tests is not performed and is limited to routine monitoring of fasting glycemia.
According to the unanimous opinion of domestic and foreign experts, the high-risk group for developing gestational diabetes includes women with significant obesity (BMI ≥30 kg/m2 ), diabetes in first-degree relatives, indications of gestational diabetes in the anamnesis or any carbohydrate metabolism disorders outside of pregnancy. To classify a woman in the high-risk group, it is enough to have one of the listed signs. These women are tested during their first visit to the doctor (it is recommended to determine the concentration of glucose in the blood on an empty stomach and a test with 100 g of glucose, see the method below).
The group with an average risk of developing gestational diabetes includes women who do not belong to the low and high risk groups: for example, with a slight excess of body weight before pregnancy, with a complicated obstetric history (large fetus, polyhydramnios, spontaneous abortions, gestosis, fetal malformations, stillbirths), etc. In this group, testing is carried out at a critical time for the development of gestational diabetes - 24-28 weeks of pregnancy (the examination begins with a screening test).
Several other clinical risk factors for the development of gestational diabetes have been reported. These clinical factors include: [ 12 ]
- Overweight (body mass index over 25)
- Decreased physical activity
- First degree relative with diabetes
- History of gestational diabetes or neonate with macrosomia, associated metabolic diseases such as hypertension.
- Low HDL
- Triglycerides over 250
- Polycystic ovary syndrome
- Hemoglobin A1C is greater than 5.7.
- Abnormalities in oral glucose tolerance test
- Any significant marker of insulin resistance (acanthosis nigricans)
- Past history of cardiovascular disease
Pathogenesis
Human placental lactogen is a hormone secreted by the placenta during pregnancy. It has a composition comparable to growth hormone and causes important metabolic changes during pregnancy to maintain the nutritional status of the fetus. This hormone is capable of inducing changes and modifications in insulin receptors. The following molecular variations appear to be associated with decreased glucose uptake by peripheral tissues:
- molecular alteration of the beta subunit of the insulin receptor,
- decreased tyrosine kinase phosphorylation,
- remodeling of insulin receptor substrate-1 and phosphatidylinositol 3-kinase.
High maternal glucose levels cross the placenta and cause fetal hyperglycemia. The fetal pancreas is stimulated in response to hyperglycemia. The anabolic properties of insulin stimulate fetal tissue growth at an increased rate.
There are reports that higher body mass index and obesity may lead to low-grade inflammation. Chronic inflammation induces the synthesis of xanthurenic acid, which is associated with the development of prediabetes and gestational diabetes. [ 15 ]
Symptoms diabetes mellitus in pregnancy
Pregestational diabetes
Symptoms in pregnant women with diabetes mellitus types 1 and 2 depend on the degree of compensation and duration of the disease and are mainly determined by the presence and stage of chronic vascular complications of diabetes (arterial hypertension, diabetic retinopathy, diabetic nephropathy, diabetic polyneuropathy, etc.).
 [ 16 ], [ 17 ], [ 18 ], [ 19 ]
[ 16 ], [ 17 ], [ 18 ], [ 19 ]
Gestational diabetes
Symptoms of gestational diabetes depend on the degree of hyperglycemia. It may manifest itself as slight hyperglycemia on an empty stomach, postprandial hyperglycemia, or the classic clinical picture of diabetes mellitus with high glycemia figures develops. In most cases, clinical manifestations are absent or nonspecific. As a rule, there is obesity of varying degrees, often - rapid weight gain during pregnancy. With high glycemia figures, complaints of polyuria, thirst, increased appetite, etc. appear. The greatest difficulties for diagnosis are cases of gestational diabetes with moderate hyperglycemia, when glucosuria and fasting hyperglycemia are often not detected.
In our country, there are no unified approaches to diagnosing gestational diabetes. According to modern recommendations, diagnosing gestational diabetes should be based on determining risk factors for its development and using glucose load tests in medium and high risk groups.
Forms
Among the carbohydrate metabolism disorders in pregnant women, it is necessary to distinguish:
- Diabetes that existed in a woman before pregnancy (pregestational diabetes) - type 1 diabetes, type 2 diabetes, other types of diabetes.
- Gestational diabetes or diabetes of pregnant women is any degree of carbohydrate metabolism disorder (from isolated fasting hyperglycemia to clinically obvious diabetes) with onset and first detection during pregnancy.
Classification of pregestational diabetes
By the degree of compensation of the disease:
- compensation;
- decompensation.
Classification of gestational diabetes
Gestational diabetes is differentiated depending on the treatment method used:
- compensated by diet therapy;
- compensated by insulin therapy.
By the degree of compensation of the disease:
- compensation;
- decompensation.
- E10 Insulin-dependent diabetes mellitus (in the modern classification - diabetes mellitus type 1)
- E11 Non-insulin-dependent diabetes mellitus (in the modern classification - diabetes mellitus type 2)
- E10(E11).0 - with coma
- E10(E11).1 - with ketoacidosis
- E10(E11).2 - with kidney damage
- E10(E11).3 - with eye damage
- E10(E11).4 - with neurological complications
- E10(E11).5 - with peripheral circulatory disorders
- E10(E11).6 - with other specified complications
- E10(E11).7 - with multiple complications
- E10(E11).8 - with unspecified complications
- E10(E11).9 - no complications
- 024.4 Diabetes in pregnancy.
Complications and consequences
A pregnant woman with diabetes and her unborn child are at increased risk of pregnancy complications such as preeclampsia, infections, obstructed labor, postpartum hemorrhage, preterm labor, stillbirth, macrosomia, miscarriage, intrauterine growth restriction, congenital anomalies, birth injuries, and death in the worst-case scenarios. Women are also at risk of long-term diabetic complications including retinopathy, nephropathy, and neuropathy.
After the 42-day postpartum period, the effects of diabetes during pregnancy can also be observed. It is estimated that 30–50% of women with a history of GDM will develop it again in subsequent pregnancies, and 50% of these women will develop type 2 diabetes within 5–10 years. In addition, children born from pregnancies with diabetes have an increased risk of developing obesity in childhood, metabolic disorders in adolescence, and type 2 diabetes in adulthood, due to the metabolic imbalances observed in utero.
Diagnostics diabetes mellitus in pregnancy
Domestic and foreign experts offer the following approaches to diagnosing gestational diabetes. The one-step approach is most cost-effective for women with a high risk of developing gestational diabetes. It involves conducting a diagnostic test with 100 g of glucose. The two-step approach is recommended for the average-risk group. With this method, a screening test with 50 g of glucose is first performed, and if it is abnormal, a 100-gram test is performed.
The screening test is performed as follows: the woman drinks 50 g of glucose dissolved in a glass of water (at any time, not on an empty stomach), and after an hour the glucose in venous plasma is determined. If after an hour the plasma glucose is less than 7.2 mmol/L, the test is considered negative and the examination is stopped. (Some guidelines suggest a glycemia level of 7.8 mmol/L as a criterion for a positive screening test, but they indicate that a glycemia level of 7.2 mmol/L is a more sensitive marker of an increased risk of gestational diabetes.) If the plasma glucose is equal to or greater than 7.2 mmol/L, a test with 100 g of glucose is indicated.
The 100 g glucose test requires a more stringent protocol. The test is performed in the morning on an empty stomach, after an overnight fast of 8-14 hours, against the background of a normal diet (at least 150 g of carbohydrates per day) and unlimited physical activity for at least 3 days before the test. You must sit during the test; smoking is prohibited. The test determines venous plasma glycemia on an empty stomach, after 1 hour, after 2 hours, and after 3 hours after exercise. Gestational diabetes is diagnosed if 2 or more glycemia values are equal to or exceed the following figures: fasting - 5.3 mmol/l, after 1 hour - 10 mmol/l, after 2 hours - 8.6 mmol/l, after 3 hours - 7.8 mmol/l. An alternative approach may be to use a 2-hour test with 75 g glucose (the protocol is similar). To establish the diagnosis of gestational diabetes in this case, it is necessary that the venous plasma glucose levels in 2 or more determinations be equal to or exceed the following values: fasting - 5.3 mmol / l, after 1 hour - 10 mmol / l, after 2 hours - 8.6 mmol / l. However, according to experts from the American Diabetes Association, this approach does not have the validity of a 100-gram sample. The use of the fourth (three-hour) determination of glycemia in the analysis when performing a test with 100 g of glucose allows for more reliable testing of the state of carbohydrate metabolism in a pregnant woman. It should be noted that routine monitoring of fasting glycemia in women at risk of gestational diabetes in some cases cannot completely exclude gestational diabetes, since the normal level of fasting glycemia in pregnant women is slightly lower than in non-pregnant women. Thus, fasting normoglycemia does not exclude the presence of postprandial glycemia, which is a manifestation of gestational diabetes and can only be detected as a result of stress tests. If high glycemia values are detected in a pregnant woman's venous plasma: more than 7 mmol/l on an empty stomach and more than 11.1 in a random blood sample, and these values are confirmed the next day, diagnostic tests are not required, and the diagnosis of gestational diabetes is considered established.
What do need to examine?
How to examine?
Who to contact?
Treatment diabetes mellitus in pregnancy
Pregnant women with diabetes mellitus are at risk for the following obstetric and perinatal complications: spontaneous abortion, gestosis, polyhydramnios, premature birth, hypoxia and intrauterine fetal death, fetal macrosomia, intrauterine growth retardation and the formation of fetal developmental abnormalities, birth trauma to the mother and fetus, high intra- and postnatal mortality. That is why the management of pregnant women with diabetes mellitus, both at the outpatient and inpatient stages, should be organized from the point of view of rational prevention and monitoring of the above complications. The main principles of rational management of pregnant women with diabetes mellitus and gestational diabetes include:
Strict glycemic control and maintenance of stable compensation of carbohydrate metabolism
Diabetes management during pregnancy involves both regular assessment of diabetes compensation by an endocrinologist (keeping a diary, determining glycated hemoglobin, adjusting diet therapy and insulin therapy) and self-monitoring of blood glucose levels by the pregnant woman herself. Self-monitoring of glycemia is performed on an empty stomach, before, 1 and 2 hours after main meals, and before bedtime. If hyperglycemia is detected after a meal, it is immediately corrected by injecting short-acting insulin. Self-monitoring of urine glucose is currently not recommended due to its low information content. A woman also self-monitors ketonuria (in the morning portion of urine, as well as with glycemia over 11–12 mmol/l), keeps a diabetes diary, where glycemia levels, insulin doses, the number of bread units, episodes of hypoglycemia, acetonuria, body weight, blood pressure, etc. are recorded.
Monitoring diabetic complications
At least once per trimester, an ophthalmologist consultation is conducted to decide on the need for laser photocoagulation of the retina. Particular attention is paid to dynamic monitoring of the kidneys. The frequency of laboratory tests is determined individually. The following scheme can be proposed as an indicative one: daily proteinuria - once per trimester, blood creatinine - at least once per month, Reberg test - at least once per trimester, general urine analysis - once every 2 weeks. Blood pressure is monitored, if necessary, antihypertensive therapy is prescribed (or adjusted).
- Prevention and treatment of obstetric complications (fetoplacental insufficiency, miscarriage, gestosis, etc.) consist of the use of progesterone preparations, antiplatelet agents or anticoagulants, membrane stabilizers, antioxidants according to the generally accepted obstetric regimens.
- Monitoring the condition of the fetus
It is performed for the purpose of timely diagnostics and treatment of such complications as malformations, hypoxia, macrosomia, intrauterine growth retardation of the fetus. At the 7th–10th week, an ultrasound scan of the fetus is performed (to determine viability, calculate the crown-rump length, and clarify the gestational age). At the 16th–18th week, an analysis is performed for serum alpha-fetoprotein (diagnosis of neural tube malformations), β-CG, and estriol. At the 16th–20th week, a repeat ultrasound scan of the fetus (diagnosis of major fetal malformations). At the 22nd–24th week, an echocardiogram of the fetus is performed to diagnose fetal cardiovascular malformations. From the 28th week, every 2 weeks, ultrasound biometry of the fetus (to assess fetal growth and compliance of its size with the gestational age), Doppler ultrasound, and assessment of the fetoplacental complex. From the 32nd week - weekly cardiotocography (more often if indicated, depending on the obstetric situation). In the later stages of pregnancy, daily registration of the fetus's motor activity by the pregnant woman herself is necessary, with data being entered into a diabetes diary.
Goals of treatment for diabetes during pregnancy
- Stable compensation of carbohydrate metabolism throughout pregnancy.
- Prevention of development and treatment of existing diabetic and obstetric complications.
Pregestational diabetes
- Target glycemic values (capillary blood): fasting - 4.0–5.5 mmol/l, 2 hours after eating < 6.7 mmol/l.
- Target HbA1c values (at least once per trimester) - within the reference values for non-pregnant women or below.
- Ketonuria is absent.
Gestational diabetes
- Target glycemic values (capillary blood): fasting - < 5.0 mmol/l, 2 hours after eating < 6.7 mmol/l.
- Target HbA1c values (at least once per trimester) - within the reference values for non-pregnant women or below.
- Ketonuria is absent.
Indications for hospitalization
Pregestational diabetes
Pregnant women with diabetes mellitus types 1 and 2 are usually recommended 3 planned hospitalizations. The first - in the early stages of gestation - for a comprehensive clinical and laboratory examination, deciding on prolongation of pregnancy, undergoing diabetes school (for women with diabetes mellitus who are not prepared for pregnancy), specifying the gestational age, compensating for diabetes mellitus. The second - at 21-24 weeks of pregnancy - at a critical time for decompensation of diabetes mellitus, to compensate for carbohydrate metabolism and prevent the progression of diabetic and obstetric complications. The third - at 32 weeks of pregnancy for further monitoring and treatment of obstetric and diabetic complications, careful observation of the fetus, determining the time and method of delivery.
Gestational diabetes
Hospitalization is indicated at the first detection of gestational diabetes for examination and selection of therapy, then in the event of worsening of the course of diabetes and for obstetric indications.
Treatment methods for diabetes during pregnancy
Pregestational diabetes
The most important measure in the event of pregnancy in women with diabetes mellitus is modification of hypoglycemic therapy. The "gold standard" of hypoglycemic therapy during gestation is intensified therapy with genetically engineered human insulins. If a woman's pregnancy was planned, then by the time the pregnancy occurs, she should already be on this type of insulin therapy. If pregnancy was not planned and occurs in a woman with type 2 diabetes mellitus taking oral hypoglycemic drugs (sulfonylurea drugs, acarbose, metformin, glitazones, glinides), then they should be discontinued and insulin therapy should be prescribed. In women with type 2 diabetes mellitus who are on diet therapy, insulin therapy is also usually necessary when pregnancy occurs. If a woman was on traditional insulin therapy (for diabetes mellitus types 1 and 2), she should be transferred to intensified insulin therapy in a regimen of five-time injections (short-acting insulin 3 times a day before main meals and medium-acting insulin in the morning before breakfast and before bedtime). Data on the use of human insulin analogues during pregnancy are currently limited (insulin lispro, insulin aspart, insulin glargine, etc.).
In the conditions of constantly changing insulin requirements during pregnancy, for timely correction of insulin doses, it is necessary to consult an endocrinologist with an analysis of the diabetes diary once every 2 weeks in the early stages, and weekly - from the 28th week of pregnancy. In this case, it is necessary to take into account the patterns of changes in insulin sensitivity and the features of insulin therapy at different stages of pregnancy and the postpartum period.
In the first trimester of pregnancy, tissue sensitivity to insulin increases, which leads to a decrease in the pregnant woman's need for insulin. The risk of hypoglycemia increases significantly, so the insulin dose should be reduced in a timely manner. However, hyperglycemia should not be allowed either, since during this period the fetus does not synthesize its own insulin, and the mother's glucose easily penetrates through the placenta into its organs and tissues. Excessive reduction in the insulin dose quickly leads to the development of ketoacidosis, which is especially dangerous, since ketone bodies easily overcome the placental barrier and have a powerful teratogenic effect. Thus, maintaining normoglycemia and preventing ketoacidosis in the early stages of pregnancy are necessary for preventing fetal developmental abnormalities.
From the 13th week of pregnancy, under the influence of placental hormones with counterinsular action, the need for insulin increases, so the dose of insulin required to achieve normoglycemia is gradually increased. During this period, the fetus already synthesizes its own insulin. With inadequate compensation of diabetes, hyperglycemia in the mother leads to hyperglycemia and hyperinsulinemia in the fetal bloodstream. Fetal hyperinsulinemia is the cause of complications such as macrosomia (diabetic fetopathy), impaired maturation of the fetal lungs, respiratory distress syndrome of the newborn, neonatal hypoglycemia.
Starting from the 32nd week of pregnancy and until the birth, the risk of hypoglycemia increases again. During this period, the insulin dose can be reduced by 20-30%. Improvement in the course of diabetes during this period of pregnancy is associated with increased glucose consumption by the growing fetus and the "aging" of the placenta.
During labor, significant fluctuations in blood glucose levels may occur. Hyperglycemia and ketoacidosis (against the background of the release of counter-insular hormones under the influence of pain and fear) and severe hypoglycemia associated with heavy physical exertion during labor may develop.
Immediately after childbirth, the need for insulin drops sharply, reaching 0-5 U per day in some women. The lowest level of glycemia occurs on the 1st-3rd day after childbirth, during which time the insulin dose should be minimal. By the 7th-10th day of the postpartum period, the need for insulin gradually returns to the level that the woman had before pregnancy.
Gestational diabetes
The first stage of gestational diabetes treatment is diet therapy combined with measured physical activity. The main principles of diet therapy are the exclusion of easily digestible carbohydrates (sugar, honey, jam, sweets, fruit juices, etc.), as well as fractional, even intake of complex carbohydrates throughout the day (3 main and 3 intermediate meals), which allows you to control postprandial glycemia and prevent hunger ketosis. The main sources of carbohydrates are cereals, pasta, unleavened bakery products, corn, legumes, potatoes, etc. The diet should be rich in proteins (1.5 g / kg of body weight), fiber, vitamins and minerals. Fats are moderately limited (to prevent excessive weight gain). A sharp restriction of the calorie content of the diet and complete fasting during pregnancy are contraindicated!
If the target glycemic values are not achieved during the diet for 1-2 weeks, insulin therapy is prescribed. Often, small doses of short-acting insulin before main meals are enough to normalize carbohydrate metabolism. However, as pregnancy progresses, the need for insulin may change. It should be especially noted that if the diet is ineffective, it is absolutely unacceptable to prescribe oral hypoglycemic drugs to pregnant women! Signs of macrosomia in ultrasound biometry of the fetus may serve as an indication for prescribing insulin therapy to a pregnant woman with gestational diabetes. Pregnant women with gestational diabetes who are on insulin therapy need to keep a diary in which the following is recorded: the results of self-monitoring of blood glucose levels (6-8 times a day), the amount of carbohydrates per meal, calculated using the bread units (BU) system, insulin doses, body weight (weekly), notes (episodes of hypoglycemia, acetonuria, blood pressure, etc.). To assess the effectiveness of any type of treatment for gestational diabetes (diet therapy, insulin therapy), the level of glycated hemoglobin is tested at least once per trimester.
Complications and side effects of treatment
In pregnant women with diabetes mellitus and gestational diabetes, who are on insulin therapy and well compensated, the occurrence of mild hypoglycemia is inevitable, which is harmless to the mother and fetus. Women should be able to independently stop mild forms of hypoglycemia to prevent the development of severe (with impaired consciousness) hypoglycemic reactions.
 [ 32 ], [ 33 ], [ 34 ], [ 35 ]
[ 32 ], [ 33 ], [ 34 ], [ 35 ]
Timing and methods of delivery
Pregestational diabetes
The term and method of delivery are determined individually. The optimal term is 37–38 weeks, the preferred method is programmed delivery through the natural birth canal. The course of labor in women with diabetes mellitus may be complicated due to the presence in most cases of fetoplacental insufficiency, gestosis, and often fetal macrosomia and polyhydramnios. It is necessary to ensure that cesarean section is performed only for obstetric indications, but in practice the frequency of operative delivery by cesarean section in women with diabetes mellitus often reaches 50% or more. Additional indications for cesarean section in diabetes mellitus may be the progression of chronic and the development of acute diabetic complications. Early delivery is undertaken in case of a sharp deterioration in the condition of the fetus, progression of gestosis, retinopathy (the appearance of multiple fresh hemorrhages in the fundus), nephropathy (development of signs of renal failure). On the night before a caesarean section, a pregnant woman with diabetes mellitus is given a regular dose of intermediate-acting insulin. On the day of the operation, subcutaneous insulin injections are discontinued and an intravenous infusion of a glucose-potassium mixture with insulin is started under glycemic control every 1–2 hours using the express method. The target glycemic level during labor or caesarean section (in capillary blood) is 4–7 mmol/l. Antibiotic therapy is used to reduce the risk of infectious complications in the postpartum period.
Gestational diabetes
Gestational diabetes itself is not an indication for cesarean section or for early delivery before the completion of 38 full weeks of gestation. The optimal time for delivery is during the 38th week of gestation (unless the obstetric situation dictates otherwise). Prolongation of pregnancy beyond 38 weeks is not indicated, as it increases the risk of macrosomia. The method of delivery is determined by obstetric indications.
Further management
Pregestational diabetes
In case of type 2 diabetes mellitus during breastfeeding, it is recommended to continue insulin therapy, since the use of oral hypoglycemic agents during lactation can cause hypoglycemia in the child. After the cessation of lactation, women with type 1 and 2 diabetes mellitus need to consult an endocrinologist to modify hypoglycemic and symptomatic therapy [prescription of modern analogues of human insulin, oral hypoglycemic agents (for type 2 diabetes mellitus), statins, etc.], as well as to continue monitoring and treatment of diabetic complications. Before discharge from the hospital (after childbirth), it is advisable to discuss possible methods of contraception.
Gestational diabetes
After delivery, 98% of women who have had gestational diabetes have normalized carbohydrate metabolism. If this does not happen, one should think about type 1 diabetes mellitus that developed for the first time during pregnancy (if the need for insulin remains) or type 2 diabetes mellitus (if insulin therapy is not required). All women who have had gestational diabetes are at increased risk of developing type 2 diabetes mellitus, so 1.5–3 months after delivery, they need to consult an endocrinologist for an accurate assessment of the state of carbohydrate metabolism (conducting an oral glucose tolerance test with 75 g of glucose) and determining the frequency of dynamic observation.
More information of the treatment
Prevention
Prevention of pregestational diabetes depends on its pathogenetic form (diabetes mellitus type 1, diabetes mellitus type 2, other types of diabetes mellitus) and is one of the most pressing and still completely unresolved problems of modern medicine.
Prevention of complications of pregestational diabetes (for mother and fetus) is based on the widespread promotion of pre-pregnancy preparation in women with diabetes. It has now been proven that pregnancy planning is the most promising direction in improving the pregnancy prognosis in women with type 1 and type 2 diabetes. The main principles of pre-pregnancy preparation include:
- informing women about the risks associated with an unplanned pregnancy against the background of poor metabolic control (high risk of malformations and loss of the fetus, complicated pregnancy, progression of chronic vascular complications of diabetes up to loss of vision and the need for hemodialysis);
- achieving strict compensation of diabetes mellitus (achieving a glycated hemoglobin level of less than 7% without increasing the frequency of hypoglycemia) at least 2–3 months before pregnancy and throughout pregnancy;
- screening and treatment of chronic diabetic complications before pregnancy;
- identification and treatment of concomitant gynecological and extragenital diseases before pregnancy.
The implementation of the basic principles of pre-gravid preparation is carried out by the following methods:
- lifestyle modification: healthy eating, smoking cessation, folic acid supplementation (4–5 mg/day), consumption of iodized salt are recommended;
- comprehensive examination and treatment by an experienced multidisciplinary team of specialists (endocrinologist, obstetrician-gynecologist, therapist, ophthalmologist, neurologist, geneticist and others);
- integration of women into diabetes management (training in diabetes school);
- contraception for the entire period of achieving diabetes compensation and treatment of concomitant pathology;
- modification of hypoglycemic and other drug therapy: in type 2 diabetes mellitus, oral hypoglycemic drugs should be discontinued and insulin therapy should be prescribed; ACE inhibitors, statins, etc. should be discontinued.
The most important points during examination by specialists of various profiles are the following. When examining the cardiovascular system, it is necessary to clarify the presence and severity of arterial hypertension, coronary heart disease, diabetic macroangiopathy, other diseases of the heart and blood vessels. A detailed examination of the kidneys should answer the question of the presence and stage of diabetic nephropathy, asymptomatic bacteriuria, chronic pyelonephritis, etc. A consultation with a neurologist is necessary for the diagnosis of sensorimotor neuropathy, various forms of autonomous diabetic neuropathy (cardiovascular, gastrointestinal, urogenital), diabetic foot syndrome. It is also necessary to assess the condition of other organs of the endocrine system: first of all, the thyroid gland. An examination of the fundus with a dilated pupil by an experienced ophthalmologist is mandatory to determine the stage of diabetic retinopathy and indications for laser photocoagulation of the retina. If such indications are detected, laser photocoagulation of the retina should be performed before pregnancy. A comprehensive examination by an obstetrician-gynecologist is necessary to assess the state of the reproductive function, the presence of specific and non-specific genital infections. If foci of infection (urogenital, odontogenic, ENT infection) are detected, it is necessary to sanitize them before pregnancy, since the presence of a chronic inflammatory process in the body complicates the compensation of diabetes mellitus.
After receiving the results of the examination, relative and absolute contraindications to carrying a pregnancy are determined in a consultative manner.
Absolute contraindications to pregnancy in diabetes are:
- severe diabetic nephropathy with proteinuria and signs of incipient chronic renal failure;
- progressive, refractory proliferative retinopathy;
- severe ischemic heart disease;
- severe autonomic neuropathy (orthostatic hypotension, gastroparesis, enteropathy, loss of ability to recognize hypoglycemia).
Relative contraindications to pregnancy in diabetes mellitus should be considered:
- decompensation of the disease in the early period of pregnancy (the development of diabetic ketoacidosis during this period increases the risk of fetal developmental abnormalities);
- a combination of diabetes mellitus with severe concomitant diseases (for example, chronic continuously recurring pyelonephritis, active tuberculosis, blood diseases, heart disease, etc.).
Prevention of gestational diabetes consists of correcting the removable risk factors for its development (primarily obesity). Prevention of complications of gestational diabetes (for the mother and fetus) consists of early detection and active treatment (expanding the indications for insulin therapy) of this disease.
Physical activity has long been known to improve glucose homeostasis through direct or indirect effects on insulin sensitivity via multiple mechanisms. For example, physical activity has independent effects on glucose disposal, increasing both insulin-mediated and non-insulin-mediated glucose disposal. [ 36 ], [ 37 ] Physical activity may also have long-term effects on improving insulin sensitivity through increases in fat-free mass. [ 38 ] Moreover, benefits in preventing or delaying the development of type 2 diabetes have been repeatedly reported among non-pregnant women. [ 39 ], [ 40 ] Thus, physical activity may have the potential to prevent GDM and its associated adverse health consequences.
Forecast
Despite the fact that pregnancy in women with diabetes mellitus is accompanied by a high risk of obstetric and perinatal complications, pregnancy planning and its rational management contribute to a significant reduction in adverse pregnancy outcomes for the mother with diabetes mellitus and her offspring.

