Medical expert of the article
New publications
Diabetes mellitus treatment
Last reviewed: 04.07.2025

All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.
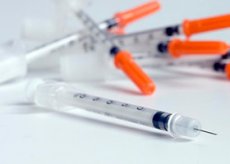
Insulin therapy aims to provide maximum compensation for diabetes mellitus and prevent the progression of its complications. Insulin treatment can be either permanent and lifelong for patients with type 1 diabetes mellitus or temporary, due to various situations, for patients with type 2 diabetes mellitus.
Indications for insulin therapy
- Type 1 diabetes.
- Ketoacidosis, diabetic, hyperosmolar, hyperlaccidemic coma.
- Pregnancy and childbirth in diabetes mellitus.
- Significant decompensation of type II diabetes mellitus caused by various factors (stressful situations, infections, injuries, surgical interventions, exacerbation of somatic diseases).
- Lack of effect from other methods of treating type II diabetes.
- Significant weight loss in diabetes.
- Diabetic nephropathy with impaired nitrogen-excreting function of the kidneys in type II diabetes mellitus.
Currently, there is a wide range of insulin preparations that differ in duration of action (short, medium and long), degree of purification (single-peak, single-component) and species specificity (human, porcine, bovine).
The Pharmaceutical Committee of the Ministry of Health of the Russian Federation recommends using only single-component preparations of human and porcine insulin for treating patients, since beef insulin causes allergic reactions, insulin resistance, and lipodystrophy.
Insulin is produced in vials of 40 U/ml and 100 U/ml for subcutaneous administration with disposable syringes specially designed for the use of insulins of the corresponding concentration of 40-100 U/ml.
In addition, insulin is produced in the form of penfills with an insulin concentration of 100 U/ml for syringe pens. Penfills can contain insulins of different durations of action and combined (short + extended action), the so-called mixtards.
Various syringe pens are produced for use by patients, allowing one to inject from 1 to 36 units of insulin at a time. The Novopen I, II, and III syringe pens are produced by Novonordisk (1.5 and 3 ml inserts), Optipen 1, 2, and 4 by Hoechst (3 ml inserts), Berlinpen 1 and 2 by Berlin-Chemie (1.5 ml inserts), Lilipen and B-D pen by Eli Lilly and Becton-Dickenson (1.5 ml inserts).
Domestic production is represented by the syringe pens "Crystal-3", "In-sulpen" and "Insulpen 2".
In addition to traditional insulins, an insulin analogue, Humalog (Eli Lilly), is also used in the treatment of patients. It is obtained by rearranging the amino acids lysine and proline in the insulin molecule. This has led to an acceleration of its sugar-lowering effect and to its significant shortening (1-1.5 hours). Therefore, the drug is administered immediately before meals.
For each patient with diabetes, a particular type of insulin is individually selected to improve overall well-being, achieve minimal glucosuria (no more than 5% of the sugar value of food) and acceptable fluctuations in blood sugar levels during the day for a given patient (no higher than 180 mg%). JS Skyler and M. L. Reeves believe that for more reliable prevention or slowing down of manifestations of diabetic microangiopathy and other late metabolic complications of diabetes, the criteria for its compensation should be more stringent. For patients prone to hypoglycemic conditions, the glucose level before meals can be 120-150 mg/100 ml.
Criteria for compensation of diabetes mellitus
Research time |
Glucose level (mg/100 ml) |
|
Ideal |
Acceptable |
|
On an empty stomach before breakfast |
70-90 |
70-110 |
Before meals during the day |
70-105 |
70-130 |
1 hour after meals |
100-160 |
100-180 |
2 hours after eating |
80-120 |
80-150 |
When selecting insulin, the severity of the disease, previously used therapy and its effectiveness should be taken into account. In outpatient settings, the criteria for choosing insulin are fasting glycemia levels, glucosuric profile data or daily glucosuria. In hospitals, there are greater opportunities for more correct insulin prescription, since a detailed examination of carbohydrate metabolism is carried out: glycemic profile (blood sugar determination every 4 hours during the day: 8-12-16-20-24-4 hours), 5-time glucosuric profile (the 1st portion of urine is collected from breakfast to lunch; the 2nd - from lunch to dinner; the 3rd - from dinner to 22:00; the 4th - from 22:00 to 6:00; the 5th - from 6:00 to 9:00). Insulin is prescribed depending on the level of glycemia and excess glucosuria.
All insulins, depending on the method of their production, can be conditionally divided into two main groups: heterologous insulins from the pancreas of cattle and pigs and homologous human insulins from the pancreas of pigs (semi-synthetic) or obtained by bacterial synthesis.
Currently, monotype highly purified insulins (monopeical and monocomponent) are produced, devoid of impurities. These are mainly porcine insulin preparations with different durations of action. They are used mainly for allergic reactions to bovine insulin, insulin resistance, lipodystrophies. Certain hopes were placed on the use of human semi-synthetic and genetically engineered insulin in medical practice. However, the expected significant differences in its sugar-lowering effect or influence on the formation of antibodies to insulin compared to monocomponent porcine insulin were not found.
Thus, at present, industrial production of various types of insulin has been established, the prolonged action of which depends on special processing and the addition of protein and zinc to them.
Patients with newly diagnosed diabetes mellitus and hyperglycemia and glucosuria that do not resolve within 2-3 days despite dietary restrictions require insulin therapy. If the patient's body weight deviates from the ideal by no more than ±20% and there are no acute stress situations or intercurrent infections, the initial insulin dose may be 0.5-1 U/(kg-day) (based on the ideal body weight) with subsequent correction over several days. Short-acting insulin can be used in the form of 3-4 single injections or a combination of short-acting and prolonged insulin. J. S. Skyler and M. L. Reeves [86] recommend prescribing insulin to patients at a dose of 0.4 U/(kg-day) even in the remission phase, and 0.6 U/(kg-day) to pregnant women (during the first 20 weeks). The insulin dose for patients with diabetes mellitus who have already been treated previously should not, as a rule, exceed, on average, 0.7 U/(kg-day) based on ideal body weight.
The availability of drugs with different durations of action in medical practice initially led to the tendency to create "cocktails" to provide a sugar-lowering effect during the day with one injection. However, this method did not allow in most cases, especially with a labile course of the disease, to achieve good compensation. Therefore, in recent years, various insulin administration regimens have been used, providing maximum compensation of carbohydrate metabolism with glycemia fluctuation limits during the day from 70 to 180 or 100-200 mg/100 ml (depending on the criteria). The insulin therapy regimens used in patients with type I diabetes mellitus are largely determined by such factors as the presence and degree of residual secretion of endogenous insulin, as well as the participation of glucagon and other counter-insular hormones in eliminating significant fluctuations in blood sugar (hypoglycemia) and the severity of the insulin response to the introduced food components, glycogen reserves in the liver, etc. The most physiological is the regimen of multiple (before each meal) insulin injections, which allows for the relief of postprandial hyperglycemia. However, it does not eliminate fasting hyperglycemia (at night), since the duration of action of regular insulin until the morning is not enough. In addition, the need for frequent insulin injections creates certain inconveniences for the patient. Therefore, the multiple insulin injection regimen is most often used to quickly achieve diabetes compensation as a temporary measure (to eliminate ketoacidosis, decompensation against the background of intercurrent infections, as a preparation for surgery, etc.). Under normal conditions, injections of regular insulin are usually combined with the introduction of a prolonged-action drug in the evening, taking into account the time of their peak action to prevent nocturnal hypoglycemia. Therefore, in some cases, the drugs "lente" and "long" are administered after the second dinner before bedtime.
The most convenient regimen for students and working patients is a twice-daily insulin administration. In this case, short-acting insulins are administered in the morning and evening in combination with intermediate- or long-acting insulins. If at 3-4 a.m. a decrease in blood sugar below 100 mg/100 ml is observed, the second injection is postponed to a later time so that the decrease in sugar occurs in the morning, when the glycemia level can be tested and food can be consumed. In this case, the patient should be transferred to a 3-times-a-day insulin regimen (in the morning - a combination of insulins, before dinner - regular insulin and before bed - extended). The insulin dose when transferring the patient to 2-times-a-day injections is calculated as follows: % of the total daily dose is administered in the morning and 1/3 - in the evening; 1/3 of each calculated dose is short-acting insulin, and 2/3 is extended. If diabetes is insufficiently compensated, the insulin dose is increased or decreased depending on the blood sugar level at a specific time of day by no more than 2-4 U at a time.
According to the onset and maximum effect of each type of insulin and the number of injections, meals are distributed throughout the day. Approximate ratios of the daily diet are: breakfast - 25%, second breakfast - 15%, lunch - 30%, afternoon snack - 10%, dinner - 20%.
The degree of compensation of diabetes mellitus during the therapy is assessed by the glycemic and glucosuric profile, the content of hemoglobin HbA 1c in the blood and the level of fructosamine in the blood serum.
Methods of intensive insulin therapy
Along with traditional insulin therapy methods, since the early 80s, a regime of multiple (3 or more) insulin injections during the day (basal-bolus) has been used. This method allows maximally reproducing the insulin secretion rhythm of the pancreas of a healthy person. It has been proven that the pancreas of a healthy person secretes 30-40 U of insulin per day. It has been established that insulin secretion in healthy people occurs constantly, but at different rates. Thus, between meals, the rate of its secretion is 0.25-1.0 U/h, and during meals - 0.5-2.5 U/h (depending on the nature of the food).
The intensive insulin therapy regimen is based on imitation of constant secretion of the pancreas - creation of a basal level of insulin in the blood by introducing long-acting or intermediate-acting insulin at 10 pm before bedtime in a dose of 30-40% of the daily dose. During the day, before breakfast, lunch and dinner, sometimes before the 2nd breakfast, short-acting insulin is introduced in the form of supplements - boluses depending on the need. Insulin therapy is carried out using syringe pens.
When using this method, the blood glucose level is maintained within 4-8 mmol/l, and the content of glycated hemoglobin is maintained within its normal values.
Intensive insulin therapy by multiple injections can be carried out only if there is motivation (the patient’s desire), active training, the ability to test glucose levels at least 4 times a day (using test strips or a glucometer) and constant contact between the patient and the doctor.
Indications for intensive therapy are newly diagnosed type I diabetes, childhood, pregnancy, absence or early stages of microangiopathy (retinopathy, nephropathy).
Contraindications for the use of this method of insulin therapy are:
- tendency to hypoglycemic conditions (if the glucose level before bed is <3 mmol/l, then nocturnal hypoglycemia occurs in 100% of cases, and if <6 mmol/l, then in 24%);
- the presence of clinically expressed microangiopathy (retino-, neuro-, nephropathy).
Side effects of intensive insulin therapy include possible worsening of diabetic retinopathy manifestations and a 3-fold increase in the risk of hypoglycemic conditions (night and asymptomatic), and weight gain.
Another method of intensive insulin therapy is the use of wearable insulin micropumps, which are dosing devices filled with short-acting insulin and injecting insulin under the skin in portions according to a predetermined program. Side effects are similar, plus possible pump failure and the risk of ketoacidosis. Micropumps have not become widespread.
The goal of intensive insulin therapy is ideal compensation of carbohydrate metabolism to prevent the development of clinical forms of late complications of diabetes mellitus, which are not subject to reverse development.
In a number of countries, the production of individual wearable devices based on the principle of diffusion pumps has been mastered, with the help of which insulin under pressure at a rate regulated depending on the need is supplied through a needle under the patient's skin. The presence of several regulators that change the rate of insulin supply allows, under the control of the glycemia level, to set the mode of its administration for each patient individually. The inconveniences of use and disadvantages of these devices include the lack of a feedback system, the possibility of bedsores despite the use of plastic needles, the need to change the area of insulin administration, as well as difficulties associated with fixing the device on the patient's body. The described diffusion pumps have found application in clinical practice, especially in the labile form of diabetes mellitus. In this case, the chamber of the diffusion pump can be filled with any type of short-acting insulin, including homologous insulin.
Other methods of treatment with human insulin, involving transplantation of the pancreas or its fragments, have not yet received widespread use due to serious obstacles caused by manifestations of tissue incompatibility. Attempts to find methods of oral administration of insulin (on polymers, liposomes, bacteria) have also failed.
Pancreatic islet cell culture transplantation
Allo- and xenotransplantation are used as an auxiliary method of treating type 1 diabetes mellitus. Allotransplantation uses microfragments of human fetal pancreatic tissue (abortion material), while xenotransplantation uses islets or isolated beta cells from newborn piglets or rabbits. Pig and rabbit insulins differ in structure from human insulins by one amino acid. Donor material is usually cultured in vitro before transplantation. Cultivation reduces the immunogenicity of islet cells. Allo- or xenogenic islets and beta cells are implanted into the spleen, liver or muscle. Most patients experience a decrease in insulin requirement. The duration of this effect ranges from 8 to 14 months. The main result of transplantation is inhibition of chronic complications of type 1 diabetes mellitus. Some patients have experienced reversal of retinopathy and neuropathy. It appears that islet transplantation should be initiated at the stage of preclinical impairment characteristic of chronic complications of diabetes.
The main therapeutic effect may be due not only to insulin, but also to C-peptide. Since there are reports indicating that long-term intramuscular administration of C-peptide to patients with type I diabetes for 3-4 months stabilizes the course of diabetes, improves kidney function and causes the reverse development of diabetic neuropathy. The mechanisms of this action of C-peptide have not yet been clarified, but stimulation of Na + -K + -ATPase in the renal tubules has been detected. It is suggested that treatment with insulin in combination with C-peptide is possible.
Research continues into non-traditional routes of insulin administration: intrarectally, by inhalation, intranasally, as subcutaneous polymer granules that are subject to biodegradation, as well as the creation of personal use devices with a feedback system.
It is hoped that the existing serious research in this area will lead in the near future to a positive solution to the most important task of radically improving insulin therapy for patients with diabetes.
Physical activity
During physical exercise, metabolic processes aimed at replenishing expended energy are intensified in the working muscles. There is an increase in the utilization of energy substrates in the form of muscle glycogen, glucose and fatty acids depending on the intensity and duration of physical activity. Energy expenditure during intense but short-term physical activity lasting for several minutes is replenished by muscle glycogen. Longer (40-60 min) and intense physical activity is accompanied by an increase in glucose utilization by approximately 30-40 times. With even longer muscle loads, fatty acids become the main energy substrate, since after 4 hours of work, glycogen reserves in the liver decrease by 75%.
The level of glycemia during intensive muscle work depends on two opposite processes: the rate of glucose utilization by muscles and the factors that ensure the entry of glucose into the blood. The main role in maintaining the normal level of glucose in the blood of healthy people is played by increased gluconeogenesis, glucogenolysis, activation of the sympathetic-adrenal system and counter-insular hormones. In this case, insulin secretion is slightly reduced. In patients with diabetes, the body's response to physical activity may vary depending on the initial level of glycemia, which reflects the degree of compensation of diabetes. If blood sugar did not exceed 16.7 mmol / l (300 mg%), then physical exercise causes a decrease in glycemia, especially in those who exercise regularly, and a decrease in the need for insulin by 30-40%. In one of the freestylers, a daily 25 km run contributed to a decrease in the previously received insulin depletion (30 U), and later - to its complete cancellation. However, it should be borne in mind that incomplete replenishment of energy expenditure, i.e. insufficient and untimely intake of carbohydrates with food before physical activity with an unchanged dose of insulin can cause a hypoglycemic state with subsequent hyperglycemia and ketoacidosis.
In patients with decompensated diabetes mellitus, if the initial level of glycemia exceeds 19.4 mmol/l (350 mg%), physical activity causes activation of counter-insular hormones and increased lipolysis, since free fatty acids become the main energy substrate for working muscles (under conditions of insulin deficiency). Increased lipolysis also promotes ketogenesis, which is why ketoacidosis often occurs during physical activity in insufficiently compensated patients with type 1 diabetes. The available literature data on the role of duration and intensity of physical activity in the course of diabetes mellitus indicate an increase in glucose tolerance due to an increase in the sensitivity of insulin-dependent tissues to the action of exogenous or endogenous insulin, which may be associated with an increase or activation of insulin receptors. However, the interdependence between the sugar-lowering effect of physical activity, caused by an increase in the body's energy expenditure, the required dose of insulin and the degree of adequate replenishment of energy due to dietary carbohydrates has not received a clear quantitative expression. This circumstance requires a cautious approach to the use of physical activity in the treatment of diabetes mellitus, especially type I.
Energy expenditure during different types of physical activity
Load type |
Energy expenditure, kcal/h |
Load type |
Energy expenditure, kcal/h |
Resting state: During meals Walk at a speed of 4 km/h Walk downhill Driving a car Playing volleyball Bowling Riding a bicycle at 9 km/h |
60 84 216 312 169 210 264 270 |
Swimming at a speed of 18 m/min Dancing Gardening work Playing tennis Skiing Carpentry work Digging the earth Two-Step Master Test Jogging |
300 330 336 426 594 438 480 492 300 |
It is important to remember that indications for increased physical activity depend not only on the degree of diabetes compensation, but also on concomitant diseases and complications. Thus, diabetic retinopathy, especially proliferative, is a contraindication, since physical exercise, causing an increase in blood pressure, can contribute to its progression (hemorrhages, retinal detachment). In patients with diabetic nephropathy, proteinuria increases, which can also adversely affect its course. In patients with type II diabetes mellitus, the presence of indications and contraindications to physical activity depends on concomitant diseases of the cardiovascular system. In the absence of contraindications to the use of physical exercise as an additional therapeutic measure, it is necessary to increase carbohydrate intake or reduce the insulin dose before physical activity. It should be remembered that subcutaneous administration of the drug over the area of working muscles is accompanied by a significant acceleration of its absorption.
Phytotherapy for diabetes
In the treatment of diabetes, herbal preparations are also used, which are decoctions, for example, from blueberry leaves, and tinctures of various herbs: zamaniha, ginseng, eleutherococcus. Official herbal sets - arphasetin and mirfazin, produced in our country and used in the form of a decoction, also give a good effect.
Arphazetin contains: blueberry (shoots) - 0.2 g, beans (pods) - 0.2 g, high zamaniha (roots) - 0.15 g, field horsetail (herb) - 0.1 g, chamomile (flowers) - 0.1 g.
Phytotherapy can only be used as an additional method in addition to the main type of treatment for diabetes.
Treatment of patients with diabetes mellitus during surgical intervention
Currently, this disease is not a contraindication for any operations. The number of patients with diabetes in surgical clinics is 1.5-6.4% of the total number of those requiring surgical intervention. Before planned operations, diabetes compensation is necessary, the criteria for which are the elimination of ketoacidosis, hypoglycemic conditions, an increase in glycemia during the day to no more than 180-200 mg% (10-11.1 mmol / l), the absence of glucosuria or its decrease to 1%. In addition, water-electrolyte metabolism disorders (dehydration or fluid retention and changes in the potassium content in the blood serum), acid-base balance (the presence of metabolic acidosis) are regulated. Particular attention in preparation for surgery should be paid to the elimination of cardiac, pulmonary and renal failure. Heart failure and myocardial infarction are the most common complications during surgery and in the postoperative period, accounting for 9% and 0.7%, respectively. Preoperative preparation includes the use of cardiac glycosides, diuretics, hypotensive and vasodilators. Correction of renal failure includes antibacterial therapy in the presence of urinary tract infection, the use of hypotensive drugs, and diet therapy. The state of the blood coagulation and anticoagulation systems also plays a significant role in preparation for surgery. Hypercoagulation syndrome is often observed in patients with myocardial infarction, cholecystitis, and diabetic gangrene, which leads to the need for direct and indirect anticoagulants. Compensation for diabetes mellitus in the preoperative period can be achieved by diet, sulfonamides, or short- or long-acting insulin. Indications for surgical intervention, the choice of anesthesia and treatment tactics for patients are determined by a council of specialists, including a surgeon, anesthesiologists, a therapist and an endocrinologist.
If the surgical intervention does not interfere with food and medication intake in the postoperative period or the restrictions are short-term, then the planned surgical intervention can be performed against the background of a diet (if glycemia during the day does not exceed 11.1 mmol / l - 200 mg% - and there is no ketoacidosis) or hypoglycemic drugs, when diabetes compensation is achieved with medium doses of sulfonamide drugs. If the highest permissible doses are necessary for compensation, and fasting blood sugar exceeds 150 mg% (8.3 mmol / l), then the patient should be transferred to insulin or add it to oral therapy.
Low-traumatic surgeries are performed against the background of diet therapy or treatment with sulfanilamide drugs (SP). Patients are operated on in the morning on an empty stomach. Patients take sulfanilamide drugs after surgery in normal doses with food. Biguanides are excluded in preparation for surgery and in the postoperative period. There were no significant differences in the course of the postoperative period and glycemic profile in patients operated on against the background of diet therapy or the use of sulfanilamide drugs, insulin.
All patients with type I diabetes, as well as type II diabetes (in case of abdominal surgeries and contraindications to food intake in the postoperative period) must be transferred to short-acting insulin before the operation. In planned surgeries, the basal glycemia level should be 6.5-8.4 mmol/l, and the highest glucose level in capillary blood should not exceed 11.1 mmol/l. Compensation of carbohydrate metabolism during and after surgery is achieved by intravenous drip administration of insulin with glucose and potassium chloride.
The total amount of glucose per day should be 120-150 g. The concentration of glucose in the administered solution is determined by the volume of liquid recommended in each specific case.
Example of calculation: the amount of glucose that is supposed to be administered during the day (for example, 120 g) and the daily dose of insulin (48 U) are divided by 24 hours to obtain the amount of glucose and insulin that must be administered intravenously every hour, i.e. for the selected example - 5 g/h of glucose and 2 U/h of insulin.
Since the operation causes a stress reaction of the patient, which involves adrenaline, cortisol, STH, glucagon, which contribute to an increase in glycemia due to the suppression of glucose utilization by insulin-dependent tissues, an increase in gluconeogenesis and glycogenolysis in the liver, the administered amount of glucose (120-150 g) is sufficient to prevent excessive hypoglycemic effect of the usual daily dose of insulin. Glycemic level is monitored every 3 hours and, if necessary, the amount of insulin or glucose administered intravenously by drip is changed. Intravenous administration of insulin and glucose during surgery is not accompanied by large fluctuations in glycemia during the day and does not cause insulin resistance, which is an advantage of this method. The described method of treatment is also used in the postoperative period until the patient is allowed to take food orally. After that, he is transferred to the regime of subcutaneous administration of simple or prolonged insulins.
In the presence of purulent processes, it is not always possible to achieve complete compensation of diabetes mellitus due to pronounced insulin resistance and intoxication. In this case, surgery can be performed at a glycemia level exceeding 13.9 mmol/l (250 mg%), and even in the presence of ketoacidosis. The method of insulin administration should be intravenous. As a rule, after surgery that helps remove the source of purulent infection from the body and the use of antibiotics, the daily need for insulin is significantly reduced and ketoacidosis disappears. Given the risk of hypoglycemia, it is necessary to continue testing blood sugar every 2-3 hours for 3-5 postoperative days.
In recent years, a standard glucose-potassium-insulin (GKI) mixture proposed by Albert and Thomas for patients with diabetes mellitus types I and II has been used in foreign surgical practice for intravenous drip administration of insulin. It consists of 500 ml of 10% glucose solution, 15 U of short-acting insulin and 10 ml/mol (7.5 ml of 10% solution) of potassium chloride. The insulin/glucose ratio is 0.3 U/g.
Infusion of this solution is started immediately before the operation and is continued for 5 hours. The rate of GKI administration is 100 ml/hour. The basal glucose level should be 6.5-11.1 mmol/l. When this variant of the mixture is administered, the patient receives 3 U of insulin and 10 g of glucose per hour. If the basal glucose level exceeds 11.1 mmol/l, the amount of insulin added to the mixture is increased to 20 U, and if basal glycemia decreases to <6.5 mmol/l, it is decreased to 10 U. With these variants, the amount of insulin administered intravenously is 4 and 2 U per 10 g of glucose, respectively. If long-term GKI infusion is required, the dose of added insulin or glucose concentration can be changed.
In addition to the initial level of glycemia, insulin resistance observed in some conditions and diseases may affect the need for insulin during surgery. If in uncomplicated diabetes mellitus the need for insulin, expressed in the insulin/glucose ratio, is 0.3 U/g, then in concomitant liver diseases and significant obesity it increases to 0.4 U/g. The greatest increase in insulin requirement is observed in severe infection, septic conditions and against the background of steroid therapy and is 0.5-0.8 U/g. Therefore, the dose of insulin added to the GKI from 15 U can, in the presence of various insulin-resistant conditions, be increased to 1 40 U.
Urgent surgical interventions associated with a strict time limit for preoperative preparation always cause great difficulties in compensating for diabetes mellitus. Before the operation, it is necessary to test blood sugar, acetone content in urine and, if the patient is conscious, to determine the dose of insulin administered. In the presence of ketoacidosis, it is important to establish the degree of dehydration (hematocrit number), determine the level of potassium and sodium in the blood (possibility of hyperosmolarity), and examine hemostasis indices. The tactics of treatment measures in this condition during preparation for an urgent operation and the operation itself are the same as during acidosis and diabetic coma. In the absence of ketoacidosis and normal arterial pressure, insulin can be administered intramuscularly (20 U at once), and then intravenously every hour at 6-8 U for 4-5 hours under the control of the glycemia level. Glucose is administered intravenously in doses of 5-7.5 g/h in the form of 5-10-20% solutions depending on the daily volume of fluid required for administration. Glycemic levels are monitored every 2-3 hours. The insulin dose is reduced to 1.5-3 U/h when blood sugar levels drop to 11.1 mmol/l (200 mg%) or less. Since insulin is partially adsorbed on the polyvinyl chloride and glass surfaces of the system used for its intravenous administration (25-50%), 7 ml of 10% albumin solution are added to prevent adsorption for every 500 ml of solution or the dose of insulin administered is increased by 50%. To prevent hypokalemia, potassium chloride is administered intravenously at 0.5 g/h for 3-4 hours. In the postoperative period (if indicated), the patient is transferred to oral nutrition and subcutaneous administration of short- and long-acting insulin.
Complications caused by insulin administration
Complications caused by insulin administration include: hypoglycemia, allergic reactions, insulin resistance, post-injection insulin lipodystrophy.
Hypoglycemia is a condition that develops in patients with diabetes mellitus when the glycemia level drops below 50 mg% (2.78 mmol/l) or when it drops very quickly with normal or even elevated values. Clinical observations indicate that such relative hypoglycemia is possible when patients feel well with high glycemia. A decrease in its level to normal leads to a deterioration in the condition: headache, dizziness, weakness. It is known that patients with labile diabetes mellitus, with frequent hypoglycemic states, develop adaptation to low blood sugar. The possibility of hypoglycemia with normal glycemia is confirmed by the rapid elimination of symptoms after the introduction of glucose. Hypoglycemia can be caused by various factors: violation of diet and nutrition regimen, physical activity, development of fatty liver infiltration, deterioration of the functional state of the kidneys, insulin overdose. Hypoglycemia is especially dangerous in patients with ischemic heart disease and brain disease. It can cause myocardial infarction or cerebrovascular accident. In addition, these conditions contribute to the progression of microangiopathies, the occurrence of fresh retinal hemorrhages, and fatty infiltration of the liver. Frequent hypoglycemia sometimes leads to organic damage to the central nervous system. Therefore, hypoglycemia prevention is of great importance for the life of a patient with diabetes mellitus. To prevent them in patients with atherosclerosis of the coronary and cerebral vessels, the criteria for diabetes compensation should be less strict: fasting glycemia not lower than 100 mg% (5.55 mmol/l), fluctuations during the day - 100-200 mg% (5.55-11.1 mmol/l). Mild hypoglycemia is eliminated by taking easily digestible carbohydrates (sugar, honey, jam). In severe cases, it is necessary to administer intravenous infusions of up to 50 ml of a 40% glucose solution, sometimes repeated, intramuscular injections of 1 mg of glucagon or adrenaline (0.1% solution - 1 ml).
Posthypoglycemic hyperglycemia (Somogyi phenomenon). Patients with type 1 diabetes, especially when treated with high doses of insulin, have acetonuria and high fasting blood sugar levels. Attempts to increase the dose of insulin do not eliminate hyperglycemia. Despite decompensation of diabetes mellitus, patients gradually gain weight. A study of daily and portion glucosuria indicates the absence of sugar in the urine in some night portions and the presence of acetone and sugar in the urine in others. These signs allow diagnosing postglycemic hyperglycemia, which develops as a result of insulin overdose. Hypoglycemia, which develops more often at night, causes a compensatory release of catecholamines, glucagon, and cortisol, which sharply increase lipolysis and promote ketogenesis and an increase in blood sugar. If the Somogyi phenomenon is suspected, it is necessary to reduce the dose of insulin administered (usually in the evening) by 10-20%, and more if necessary.
The Somogyi effect is differentiated from the phenomenon of "dawn", which is observed not only in patients with diabetes mellitus, but also in healthy people and is expressed in morning hyperglycemia. Its genesis is due to hypersecretion of growth hormone at night and before dawn (from 2 to 8 am). Unlike the Somogyi phenomenon, morning hyperglycemia is not preceded by hypoglycemia. The phenomenon of "dawn" can be observed in patients with both type I and II diabetes (against the background of diet therapy or treatment with hypoglycemic drugs).
Allergic reactions to insulin administration can be local and general. The former involve the appearance of hyperemia and compaction at the site of insulin administration, which can persist from several hours to several months. A general reaction manifests itself in the form of urticarial generalized rash, weakness, itching, edema, gastrointestinal disorders, and increased body temperature. If an allergy is present, antihistamine therapy should be prescribed, the type of insulin should be changed, and monopeak, monocomponent preparations of porcine or human insulin should be prescribed. Prednisolone may be prescribed at 30-60 mg every other day (in severe cases) for 2-3 weeks with its gradual withdrawal.
Post-injection insulin lipodystrophies occur in 10-60% of patients receiving the drug and develop primarily in women. They occur during treatment with all types of insulin, regardless of the drug dosage, compensation or decompensation of diabetes mellitus, more often after several months or years of insulin therapy. At the same time, cases have been described that arose after several weeks of insulin treatment. Lipodystrophies occur in the form of a hypertrophic form (increased fat formation in the subcutaneous fat tissue at the injection site), but more often - in the form of fat atrophy (atrophic form).
Lipoatrophy is not only a cosmetic defect. It leads to impaired insulin absorption, pain that increases with changes in barometric pressure. There are several theories of lipodystrophy, considering them as a consequence of one or several factors: inflammatory reaction, response to mechanical destruction of cells, poor quality of insulin preparations (admixture of pancreatic lipase, phenol, antigenic properties, low pH), low temperature of the administered preparation, alcohol penetration into the subcutaneous tissue. Some researchers adhere to the neurogenic-dystrophic concept of impaired local regulation of lipogenesis and lipolysis, while others assign the main role to immune mechanisms. Highly purified (monocomponent) porcine insulin and, especially, human insulin give a good effect. The duration of therapy depends on the size, prevalence of lipodystrophy and the effect of treatment. In the prevention of lipodystrophy, it is of great importance to change the injection sites of insulin (some authors suggest using special films with perforated holes), to reduce mechanical, thermal and chemical irritants during its administration (administering insulin warmed to body temperature, preventing alcohol from entering it, the depth and speed of administration of the drug).
Insulin resistance, as a complication of insulin therapy, was caused by the use of poorly purified beef insulin preparations, when the daily requirement sometimes reached several thousand units per day. This forced the creation of industrial insulin preparations containing 500 U/ml. The high need for insulin was due to the high titer of antibodies to beef insulin and other components of the pancreas. At present, when using monocomponent human and porcine insulin, insulin resistance is more often caused by the action of counter-insular hormones and is temporary in patients with type I diabetes. This type of insulin resistance is observed in stressful situations (surgery, trauma, acute infectious diseases, myocardial infarction, ketoacidosis, diabetic coma), as well as during pregnancy.
Immunological resistance to insulin may occur in rare conditions and diseases even against the background of the introduction of human insulin. It may be caused by defects at the prereceptor (antibodies to the insulin molecule) and receptor (antibodies to insulin receptors) levels. Insulin resistance caused by the formation of antibodies to insulin occurs in 0.01% of patients with type I diabetes mellitus, long-term treated with insulin, but may also develop several months after the start of insulin therapy.
In some cases, with high titers of insulin antibodies, it is possible to eliminate the increasing hyperglycemia only by administering 200 to 500 units of insulin per day. In this situation, it is recommended to use insulin sulfate, to which insulin receptors have a higher affinity compared to insulin antibodies. Sometimes insulin resistance takes on a wave-like character, i.e. hyperglycemia is replaced by severe hypoglycemic reactions within a few days (as a result of the rupture of the bond between insulin and antibodies).
True insulin resistance can be observed in acantosis nigricans. generalized and partial lipodystrophy, when the cause is the formation of antibodies to insulin receptors. Glucocorticoids are used in the treatment of immunological insulin resistance in doses of 60-100 mg of prednisolone per day. The effect of treatment is manifested not earlier than 48 hours after the start of therapy.
Another cause of insulin resistance is degradation or impaired absorption of insulin. In this case, with increased protease activity, subcutaneous administration of large doses of insulin does not have a sugar-lowering effect due to insulin degradation. At the same time, intravenous administration of insulin has an effect in normal doses. Malabsorption of insulin can be caused by infiltrates, impaired blood supply in the areas of insulin injections, and the presence of lipodystrophy. Frequent changes in the sites of subcutaneous administration are recommended as a preventive measure against insulin malabsorption.
In case of insulin resistance associated with excessive production of somatotropic hormone, glucocorticoids and other counter-insular hormones, it is necessary to treat the underlying disease.
Insulin edema. In patients with type I diabetes mellitus, fluid retention is observed at the beginning of insulin therapy or during the administration of large doses of the drug, which is caused by a significant decrease in glucosuria and, consequently, fluid loss, as well as the direct effect of insulin on sodium reabsorption in the renal tubules. With a decrease in the dose, the edema usually disappears.
Visual impairment. Insulin therapy sometimes causes a change in refraction due to deformation of the lens curvature. In decompensated diabetes and high hyperglycemia, the accumulation of sorbitol in the lens with subsequent fluid retention contributes to the development of myopia or weakens hyperopia. After a decrease in glycemia under the influence of insulin, the swelling of the lens decreases, and after some time, refraction is restored to previous values.
Treatment of complications of diabetes mellitus
Prevention and treatment of complications of diabetes mellitus primarily consist of maximum compensation of diabetes with a decrease in the level of glycemia during the day to 10-11.1 mmol / l (180-200 mg%) by multiple injections of short-acting insulin or 2-3-time administration of prolonged insulin in combination with short-acting insulin in type I diabetes, or by diet therapy, the purpose of which is to normalize body weight, or a combination of diet therapy, if it is ineffective, with oral hypoglycemic drugs. The tendency to prescribe insulin to patients with type II diabetes for the purpose of treating diabetic retinopathy and neuropathy is unfounded, since the indicated clinical syndromes develop in insulin-independent tissues, and the introduction of insulin contributes to obesity, hypoglycemic states (provoking the appearance of hemorrhages in retinopathy) and insulin resistance.
Treatment of diabetic neuropathy
In case of severe pain syndrome, analgesics and sedatives are prescribed. In some cases, it is necessary to resort to promedol and pantopon. A good effect is achieved by using vitamin B12, ascorbic acid, diphenin, the metabolic drug dipromonium in injections or tablets. Clinical trials of sorbinil and its domestic analogue - isodibut, used in tablets of 0.5 g up to 3 times a day, allow us to hope for the successful action of pathogenetic therapy. At the same time, physiotherapeutic procedures are recommended.
In the presence of clinical syndromes characteristic of vegetative (autonomous) neuropathy, additional therapeutic measures are used. In the treatment of orthostatic hypotension, mineralocorticoid drugs are used: DOXA in injections, fluorohydrocortisone in doses of 0.0001-0.0004 g per day. Bandaging the legs with an elastic bandage to reduce the venous blood volume gives a good effect.
In gastropathy, cholinomimetics, cholinesterase inhibitors, metoclopramide are used, which increase the tone and motor activity of the smooth muscles of the stomach and have an antiemetic effect. In severe cases, gastric resection is performed.
Atony of the bladder is often combined with ascending urinary tract infection, therefore treatment should include antibiotics according to the sensitivity of the bacterial flora. Catheterization of the bladder should be avoided. Anticholinesterase drugs are used in therapy, and, if necessary, partial resection of the bladder is used.
In case of neuroarthropathy, the main treatment methods are prevention and removal of calluses, treatment of neurotrophic ulcers, and the use of orthopedic shoes.
A new method in the treatment of patients with type II diabetes is the use of interval hypoxic training. The treatment is carried out using a hypoxicator (a device that supplies air with reduced oxygen content at certain intervals for inhalation). Gradually, the number of cycles per session increases from 3 to 10. The procedure is carried out daily, 15-20 sessions are recommended for the course of treatment.
The conducted studies have shown that the use of interval hypoxic training significantly improves the clinical course of diabetes mellitus, reduces the manifestation of diabetic neuropathy, has a positive effect on metabolic indices, tissue diffusion, parameters of central, intracardiac hemodynamics, oxygen-transport function of blood and increases resistance to hypoxia.
Treatment of retinopathy
Treatment of retinopathy, in addition to compensation for diabetes mellitus, includes the elimination of hemorheological disorders, the use of antihypertensive, lipid-lowering drugs and vitamin therapy.
Laser therapy is used to eliminate hemorheological disorders.
At the non-proliferative stage, focal laser therapy is recommended to eliminate macular edema. At the pre-proliferative stage, panretinal photocoagulation is performed, and during the proliferative stage, panretinal photocoagulation and, if necessary, vitrectomy. At the last stage, termination of pregnancy is necessary.
To prevent the progression of the process, antihypertensive therapy is used (ACE blockers, calcium, selective beta-blockers in combination with diuretics), lipid-lowering drugs depending on the nature of hyperlipidemia, as well as B vitamins, ascorbic acid, and ascorutin.
In proliferating retinopathy, the main treatment method is laser photocoagulation, which helps eliminate neovascularization, retinal hemorrhages, and prevent retinal detachment. If hemorrhage occurs in the vitreous body, vitrectomy surgery is used, i.e., its removal and replacement with a saline solution. Hypophysectomy surgery or the introduction of radioactive yttrium into the sella turcica is practically not used to treat retinopathy. Treatment of the disease is carried out jointly with an ophthalmologist who monitors the patient every six months.
Treatment and prevention of diabetic nephropathy
Treatment of the clinical form of diabetic nephropathy (DN) at the stages of severe diabetic nephropathy (proteinuria) and chronic renal failure (uremia) is aimed at eliminating arterial hypertension, electrolyte disturbances, hyperlipidemia, urinary tract infection and improving the nitrogen-excreting function of the kidneys.
The stage of severe diabetic nephropathy is characterized by the appearance of proteinuria over 0.5 g/day, microalbuminuria over 300 mg/day, arterial hypertension, hyperlipidemia and a combination with diabetic retinopathy, neuropathy, and coronary heart disease. Treatment at this stage of diabetic nephropathy is aimed at preventing chronic renal failure.
Compensation of carbohydrate metabolism
Maximum compensation of carbohydrate metabolism in patients with type I diabetes mellitus is achieved through intensive insulin therapy (multiple injections of short-acting insulin) or a combination of prolonged-acting and short-acting insulins. Patients with type II diabetes are transferred to glufenorm or dibotin, and if there is no sufficient effect, to insulin or a combination with the above drugs to eliminate the nephrotoxic effect of other sulfanilamide drugs and their metabolites.
Antihypertensive therapy slows down the decrease in SCF and reduces proteinuria. They try to maintain blood pressure at a level not exceeding 120/80 mm Hg. For this purpose, ACE inhibitors (captopril, enalapril, ramipril, etc.), cardioselective beta-blockers, calcium antagonists (nifeditin, veropamil, riodipine, etc.), alpha-blockers (prazosin, doxazosin) are used. The most effective) is considered to be a combination of captopril or enalapril with hypothiazide.
Arterial hypertension in patients is largely caused by hypervolemia due to sodium retention, in connection with which complex therapy involves limiting table salt to 3-5 g per day, diuretics, mainly potassium-sparing ones, since hyperkalemia is often observed in patients.
Hypolipidemic therapy helps to reduce proteinuria and the progression of the pathological process in the kidneys.
Since various types of hyperlipidemia (hypercholesterolemia, hypertriglyceridemia and mixed form) are observed in 70-80% of patients, a hypocholesterol diet is used in treatment, as well as resins, nicotinic acid, statins, fibrates or a combination of them.
A low-protein diet involves limiting protein to 0.8 g/kg of body weight. In the presence of obesity - hypocaloric and moderate physical activity (if ischemic heart disease is excluded).
Elimination of urinary tract infection. Given the high frequency of cystitis, atypical pyelonephritis, asymptomatic bacteriuria, it is advisable to periodically conduct a general urine analysis, and if necessary - according to Nechiporenko. In accordance with the urine culture data, regularly conduct antibacterial therapy. Concomitant pyelonephritis worsens the functional state of the kidneys and can cause interstitial nephritis.
 [ 6 ], [ 7 ], [ 8 ], [ 9 ], [ 10 ]
[ 6 ], [ 7 ], [ 8 ], [ 9 ], [ 10 ]
Treatment at the stage of chronic renal failure (uremia)
Progression of the proteinuria stage (severe diabetic nephropathy) leads to chronic renal failure. An increase in the creatinine level in the blood from 120 to 500 μmol/l corresponds to the stage of the process at which conservative therapy is possible.
Compensation of carbohydrate metabolism is complicated by the fact that patients may experience hypoglycemia due to a decrease in the need for insulin, a decrease in the degradation of insulin by the renal enzyme insulinase, and an increase in the duration and circulation of the administered insulin. Patients with type I diabetes mellitus are shown intensive insulin therapy with frequent glycemia monitoring for a timely reduction in the required insulin dose.
- Low-protein diet. Patients are recommended to reduce protein to 0.6-0.8 g/kg of body weight and increase the carbohydrate content in the diet.
- Antihypertensive therapy. All drugs used to treat the stage of severe diabetic nephropathy. ACE inhibitors are used when the creatinine level does not exceed 300 μmol/l.
- Correction of hyperkalemia. Exclude potassium-rich foods from the diet. With high hyperkalemia, an antagonist is administered - 10% calcium gluconate solution, and ion-exchange resins are also used. If the cause of hyperkalemia is hyporeninemic hypoaldosteronism (with low blood pressure), then fluorohydrocortisone (cortinef, florinef) is used in small doses.
- Treatment of nephrotic syndrome. This condition is characterized by proteinuria >3.5 g/day, hypoalbuminemia, edema and hyperlipidemia. Treatment includes: infusion of albumin solutions, furosemide 0.6-1 g/day, hypolipidemic drugs.
- Correction of phosphorus-calcium metabolism. Hypocalcemia (result of decreased synthesis of vitamin D 3 in the kidneys) is the cause of secondary hyperparathyroidism and renal osteodystrophy. Treatment involves a diet with limited phosphorus, adding calcium preparations and vitamin D 3.
- Enterosorption in the form of activated carbon, ion exchange resins, minisorb and others is used to remove toxic products from the intestines.
- Treatment of chronic renal failure at the terminal stage. Hemodialysis or peritoneal dialysis is prescribed when the SCF decreases to 15 ml/min and the creatinine level increases to >600 μmol/l.
- Kidney transplantation is indicated when the SCF is <10 ml/min and the blood creatinine level is >500 μmol/l.
Prevention of diabetic nephropathy
Since traditional methods of treating diabetes mellitus do not prevent the progression of diabetic nephropathy at its clinical stages, there is a need to prevent diabetic nephropathy at its preclinical stages.
According to the classification, the first 3 stages of diabetic nephropathy are preclinical. Preventive measures, in addition to ideal compensation of carbohydrate metabolism, include normalization of intrarenal hemodynamics (elimination of intraglomerular hypertension) by prescribing ACE inhibitors in small doses, and at stage III - elimination of hyperlipidemia and prescription of a diet with a protein content of no more than 1 g/kg of body weight.
Recently, the search for factors preventing the development of diabetic nephropathy in patients with type II diabetes mellitus has been ongoing. It is known that mortality from uremia among patients with type II diabetes mellitus is an order of magnitude lower than in type I diabetes mellitus. Of particular note is the report by L. Wahreh et al. (1996) that intravenous infusion of C-peptide in physiological doses for 1-3 hours normalizes the glomerular filtration rate in patients with type I diabetes, and daily intramuscular injections of L-peptide for 3-4 months stabilize the course of type I diabetes and improve renal function. It has been established that C-peptide stimulates Na + -K + -ATPase in the renal tubules. It is possible that C-peptide has a protective property in relation to diabetic nephropathy, given that the main pathophysiological difference between diabetes mellitus type I and diabetes mellitus type II is the practical absence of C-peptide.
Treatment of necrobiosis lipoidica
The best results were obtained with subcutaneous administration of glucocorticoid drugs into the zone bordering the affected area or by electrophoresis and phonophoresis with hydrocortisone succinate. Also effective is a combination of dipyridamole 0.0025 g 3-4 times a day with aspirin, which helps inhibit platelet aggregation and the formation of microthrombi. Locally, lotions with 70% dimexine solution and insulin are used. In case of ulcer infection, antibiotics are used.
Prevention and treatment of heart disease
First of all, prevention of heart damage consists of maximum compensation of diabetes mellitus with a decrease in glycemia to a level not exceeding 11.1 mmol/l (200 mg%) during the day, by multiple injections of small doses of insulin or 2-time administration of prolonged insulins for type I diabetes.
Literature data indicate that good compensation of diabetes mellitus improves the functional capacity of the myocardium by normalizing metabolic processes in the heart muscle. At the same time, it is necessary to avoid chronic overdose of insulin, which causes hyperinsulinemia. In the prevention and warning of coronary atherosclerosis, the elimination of such risk factors as hypertension and hyperglycemia also plays a role. Both are more pronounced in patients with obesity, and therefore limiting the daily caloric intake of food plays a major role in eliminating these additional risk factors for atherosclerosis.
Increased blood pressure in patients with diabetes mellitus is caused by a combination with hypertension or diabetic nephropathy, which is why the treatment tactics have some peculiarities. Patients often experience sodium retention in the body and hypervolemia caused by activation of the renin-angiotensin system, plasma hyperosmolarity, or insulin administration (in patients with type I diabetes).
As is known, under the influence of increased plasma renin activity, the formation of angiotensin I increases, as well as angiotensin II with the participation of angiotensin-converting enzyme (ACE). Angiotensin II has a dual effect - both vasoconstrictive and stimulating the secretion of aldosterone. Therefore, in the combination of diabetes mellitus and hypertension, drugs that block ACE (captopril, enalapril, lisinopril, ramipril, pirindapril, etc.) are widely used. In addition to ACE antagonists, angiotensin II receptor blockers (losartan, aprovel) are also used.
In the presence of tachycardia or heart rhythm disturbances in hypertension, selective adrenobeta blockers (atenolol, metoprolol, cordanum, bisoprolol, etc.) are used. It is not recommended to prescribe these drugs to patients with diabetes mellitus with a tendency to hypoglycemia, since they inhibit the sympathoadrenal response to hypoglycemia, which is the main clinical manifestation of hypoglycemia.
The hypotensive effect of calcium antagonists is due to the relaxing effect on the myofibrils of arterioles and a decrease in the resistance of peripheral vessels. In addition, these drugs improve coronary blood flow, i.e., they have an antianginal effect in the presence of coronary heart disease.
In the treatment of patients, selective calcium blockers of the verapamil (isoptin), nifedipine (corinfar) and diltiazem (norvasc) groups are used, which do not significantly affect carbohydrate metabolism.
In the absence of a sufficient hypotensive effect from ACE blockers, a combination with adrenobeta blockers or calcium antagonists is possible. It should be noted that ACE and calcium blockers have a nephroprotective effect and are used in small doses at the initial stages of arterial hypertension.
All antihypertensive drugs in the treatment of patients are combined with a restriction of table salt in the diet to 5.5-6 g, as well as with diuretics. Potassium-sparing drugs are not indicated for patients with diabetic nephropathy accompanied by hyperkalemia (hyporeninemic hypoaldosteronism).
The use of thiazine diuretics often causes impaired glucose tolerance by suppressing insulin release. However, the degree of increase in glycemia may vary, which generally does not prevent their use.
In the presence of orthostatic hypotension, methyldopa, prazosin and reserpine should be used with caution, since they may aggravate the manifestations of orthostatic hypotension.
Potassium-sparing diuretics (aldactone, triampterene, veroshpiron) are used together with ACE blockers, which helps to eliminate sodium retention and the tendency to hypokalemia as a result of blocking the action of aldosterone in the renal tubules.
Treatment of hypertension in diabetes mellitus should begin as early as possible, and blood pressure should preferably be maintained at levels not exceeding 130/80 mm Hg.
Correction of hyperlipidemia, which is one of the additional causes that aggravate the course of atherosclerosis, also plays an important role in the prevention and warning of its progression. To do this, it is necessary to eliminate obesity, hypothyroidism and kidney disease, and to give up alcohol. Hyperlipidemia of types IV, V and occasionally I can be treated by limiting fats in the diet (in the presence of chylous serum VLDL - very low density lipoproteins). With an increase in the level of LDL (low density lipoproteins), which consist of 75% cholesterol, a diet is recommended with a limitation of products containing it (no more than 300 mg / day), adding products with a high content of unsaturated fats and soy protein to the diet. Cholestyramine, polysponin, tribusponin inhibit the absorption of cholesterol in the intestine. Miscleron and cytamifen delay the synthesis of cholesterol and reduce the level of triglycerides. Drugs that accelerate lipid metabolism and their elimination from the body include bile acid resins, linetol, arachiden, heparinoids, guareme and some vitamins (nicotinic acid, pyridoxine), as well as lipotropic substances (methionines, choline chloride).
In patients with ischemic heart disease, it is recommended to use fast-acting (nitroglycerin) and prolonged-action (nitrong, sustak, trinitrolong, erinit, nitrosorbide) nitrates, the effect of which is associated with relaxation of the smooth muscles of the venous vessels, a decrease in venous inflow to the heart, unloading the myocardium and restoring blood flow in the myocardium, as well as with increased synthesis of prostacyclins in the vascular wall. Adrenergic blockers (trazicor, cordarone, cordanum) are also used in the treatment of ischemic heart disease.
Treatment of acute myocardial infarction is carried out by conventional means. Intravenous lidocaine is recommended to reduce the risk of ventricular fibrillation, which often occurs in patients with diabetes mellitus. Since hyperglycemia increases in most cases during acute myocardial infarction in patients with diabetes, it is advisable (if necessary) to administer small doses of regular insulin in 3-4 injections against the background of the main therapy with oral sulfanilamide drugs. There is no need to transfer patients with type II diabetes from oral drugs to insulin, since this is often accompanied by severe insulin resistance. A combination of oral (sulfanilamide) drugs with insulin prevents this complication of insulin therapy and has a milder effect on the level of glycemia, preventing hypoglycemic reactions. Daily glycemia should be maintained within 8.33-11.1 mmol/l (150-200 mg%).
The most effective method of treating diabetic cardiomyopathy and autonomic cardiac neuropathy is maximum compensation of diabetes mellitus, its inherent metabolic disorders and prevention of diabetic microangiopathy progression. Trental, complamine, curantil, prodectin, carmidine are used periodically in 2-3 month courses to improve microcirculation. Inosie-F, riboxin, cocarboxylase, B and C vitamins are used in combination therapy. In case of signs of autonomic neuropathy, a diet rich in myoinositol, anticholesterase drugs, adenyl-50, dipromony are recommended in the form of a course of treatment for 2-3 months per year. Since sorbitol accumulation in the nervous tissue plays a significant role in the pathogenesis of diabetic neuropathy, great hopes are placed on the use of aldose reductase inhibitors (sorbinil, isodibut), which are undergoing clinical trials.

