Alcaptonuria - congenital enzymatic pathology
Last reviewed: 23.04.2024

All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.
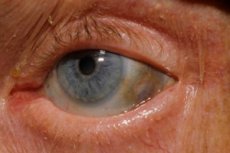
One of the very rare metabolic disorders - alkaptonuria - refers to congenital abnormalities in the metabolism of the amino acid tyrosine.
Also, this syndrome can be called homogentisate oxidase deficiency, homogentisinuria, hereditary ochronosis, or black urine disease. [1]
Epidemiology
According to statistics, there are no more than nine cases of alkaptonuria per 1 million of the population. And in most European countries - one case per 100-250 thousand live births.
Among European countries, the exception is Slovakia (especially the relatively small north-western region), where the prevalence of alkaptonuria is one case in 19 thousand newborns. In all likelihood, this is due to the fact that among the families of Slovak Roma living there, the level of inbreeding (marriages between cousins) is the highest in Europe: 10-14%. [2]
Causes of the alkaptonuria
The exact causes of alkaptonuria, as a congenital disorder of catabolism (metabolic breakdown) of the aromatic (homocyclic) α-amino acid tyrosine, have been established: this type of metabolic disorder is a consequence of homozygous or complex heterozygous mutations of one of thousands of genes on chromosome 3, more precisely, the HqG21 gene at locus 3 -q23 on the long arm of the chromosome. This gene encodes the nucleotide sequences of the liver enzyme homogentisate-1,2-dioxygenase [3](also called homogentisic acid oxidase or homogentisate oxidase), an iron-containing metalloprotein required for one of the stages of tyrosine breakdown in the body. [4], [5]
Thus, alkaptonuria is a defect in the enzyme homogentisate-1,2-dioxygenase, more precisely, the result of its genetically determined deficiency or complete absence. [6]
Being a congenital fermentopathy, alkaptonuria is inherited as an autosomal recessive trait, that is, in order for alkaptonuria to occur in children, both parents must have a modified gene for this enzyme, since each of them transmits to the child only one copy of the gene from the two available.
According to the latest data, there are more than two hundred variants of the HGD gene modification, and missense mutations, translocation and splicing are most often observed.
Risk factors
The only risk factor for the development of this congenital fermentopathy is its family history and the inheritance of two modified copies of the HGD gene, if the parents do not show alkaptonuria (the risk of transmission of the anomaly is 25%), or one of the parents has this disorder. [7]
Pathogenesis
The most important role tyrosine plays in the synthesis of proteins, the production of chromoproteins - the skin pigment melanin, as well as thyroid hormones and catecholamine neurotransmitters.
The mechanism for regulating the amount of tyrosine in cells is very complex, and the body normalizes its excess content by splitting. The process of catabolism of tyrosine, like all aromatic amino acids, is multistep and proceeds in several stages. Moreover, each stage of the metabolic decomposition of tyrosine takes place with the participation of a certain enzyme and the formation of an intermediate compound.
So, first, the amino acid is cleaved to para-hydroxyphenylpyruvate, which turns into an alkapone - 2,5-dihydroxyphenylacetic or homogentisic acid. Further, the alkapone should be transformed into maleacetic acid, but this does not happen. [8]
And the pathogenesis of alkaptonuria consists in stopping the biochemical reactions of tyrosine catabolism at the stage of formation of homogentisic acid: there is simply no necessary enzyme to break it down - homogentisate oxidase.
Homogentisic acid is not used by the body and can accumulate with excretion through the kidneys. In addition, it is oxidized to benzoquinoacetate (benzoquinoneacetic acid), which, by binding with molecules of tissues and body fluids, forms biopolymer compounds colored like melanin.
The accumulation of these intermediate products in the tissue leads to disruption of the collagen structure of the cartilaginous tissue, due to which its elasticity decreases - with the appearance of many clinical signs of alkaptonuria and the development of complications.
Symptoms of the alkaptonuria
Alcaptonuria in newborns and infants is manifested by darkening of the urine. On contact with air, the color of urine on diapers, diapers and underwear turns dark brown; this is due to the accumulation and release of homogentisic acid, which is oxidized to benzoquinoacetate. [9]
In the absence of other symptoms, alkaptonuria in children at an early age is often not recognized in a timely manner, since after urination the urine may darken after a few hours. According to some reports, in the conditions of clinics, only one fifth of children under 12 months of age who were born with this fermentopathy are detected. Therefore, it is very important for parents to pay attention to caring for the baby.
In addition, early signs include pigmentation (bluish gray) of the sclera of the eye and the cartilage of the auricles and nose, often referred to as ochronosis. [10]
Over time, other symptoms appear:
- severe pigmentation of the skin on the cheekbones, armpits and genitals;
- dyeing clothes on contact with sweating parts of the body;
- attacks of general weakness;
- hoarse voice.
It should be borne in mind that alkaptonuria and ochronosis , as noted above, are synonymous names for the same violation of tyrosine catabolism.
Maple syrup disease and alkaptonuria. Congenital maple syrup disease or leucinosis also refers to metabolic disorders, has the same type of inheritance, and even mutations occur on the same chromosome, but relate to the gene encoding the enzyme complex of branched α-keto acids dehydrogenase. Because of this, the body cannot break down certain protein components, in particular the amino acids leucine, isoleucine and valine. In this condition, urine (and earwax) has a sweet smell; in addition, in the clinical picture of this type of organic acidemia, hypopigmentation, fluctuations in blood pressure, convulsions, vomiting and diarrhea, a drop in blood glucose levels, ketoacidosis, hallucinations, etc. Are observed. The mortality rate in children is quite high; in adults without treatment, coma and death may occur due to cerebral edema.
Albinism and alkaptonuria "unite" only tyrosine. Albinism , including oculocutaneous albinism, is caused by genetic mutations that affect the production of the pigment melanin. Congenital changes are noted in the TYR gene on chromosome 11 (11q14.3), which encodes tyrosinase, a copper-containing enzyme in melanosomes that is necessary for the formation of skin pigment based on the products of tyrosine metabolism. This disease is much more common than alkaptonuria.
Complications and consequences
The consequences and complications of alkaptonuria caused by the action of the intermediate tyrosine metabolites - homogentisic and benzoquinoneacetic acids - appear due to the deposition of reactive pigmented polymers, the destruction of collagen fibrils and the deterioration of the cartilage condition (with a decrease in their resistance to mechanical stress).
Years later, in adulthood, degenerative arthritis and osteoarthritis of large joints (hip, sacroiliac and knee) develop; narrowing of the intervertebral spaces (especially of the lumbar and thoracic spine) - with calcification and the formation of osteophytes; the tissue density of the subchondral bone plates decreases, and the underlying bones can undergo pathological remodeling with the formation of growths and deformation. [11]
Damage to the heart valves (aortic and mitral) and coronary arteries, with signs of coronary heart disease, as well as the formation of kidney and prostate stones, due to the same calcification, may occur. [12], [13]
Diagnostics of the alkaptonuria
Usually, the diagnosis of congenital metabolic disorders is based on the study of body fluids.
On the basis of what tests and what reactions can alkaptonuria be diagnosed? Urine tests are required - for detecting homogentisic acid and determining its level (normal - 20-30 mg per day, increased - 3-8 g). A urine sample is examined by gas chromatography or mass spectrometry using liquid chromatography; a screening test for the presence of ferric chloride in the urine is possible. [14]
There is also a quick diagnostic method - determination of alkapton in dried urine spots on paper (by color intensity).
When clarifying the diagnosis, instrumental diagnostics (X-ray) involves the identification of X-ray signs of osteoarthritis and other articular pathologies in patients.
The diagnosis is confirmed by such molecular genetic methods as diagnostics of hereditary diseases, such as genetic testing and DNA sequencing. [15]
Differential diagnosis
Differential diagnosis includes hemochromatosis and acute liver failure of newborns, melaninuria, acute intermittent porphyria, hemophagocytic lymphohistiocytosis, primary mitochondrial pathology, rheumatoid arthritis, ankylosing spondylitis.
Who to contact?
Treatment of the alkaptonuria
The main treatment for alkaptonuria is the ingestion of large doses (at least 1000 mg per day) of ascorbic acid. In children, this increases the excretion of homogentisic acid in the urine, and in adults it reduces the level of its derivative, benzoquinoneacetic acid, in the urine and slows down its binding to the connective tissue structures of the joints and collagen. [16]
In Western European clinics, the drug Nitizinone (Orfalin) is being tested, a drug of a group of metabolites that inhibits the second stage of tyrosine catabolism: the transformation of para-hydroxyphenylpyruvate into homogentisic acid. However, the use of this pharmacological agent leads to the accumulation of tyrosine and can cause serious side effects, including corneal opacity and photophobia, nose and stomach bleeding, liver failure, changes in the blood, etc. However, in the United States, Nithizinone is approved by the FDA for the treatment of tyrosinemia I type. [17], [18]
As such, physiotherapy - exercise therapy to increase muscle strength and increase joint mobility, balneo and peloidotherapy to limit pain - is performed for joint problems caused by alkaptonuria.
Although tyrosine is not only supplied with food, but also produced in the body, patients with alkaptonuria are recommended a diet low in protein and limiting the intake of foods rich in tyrosine, primarily beef and pork, dairy products (especially cheeses), legumes, nuts, etc. Seeds.
Prevention
Prevention of gene mutations is impossible, and to prevent the birth of children with a high risk of congenital disorders, there is medical and genetic counseling, which is necessary before the planned pregnancy of those married couples in whose family history there are diseases of a hereditary nature. [19]
Forecast
There are very few fatalities in the course of alkaptonuria, and death can be caused by serious complications affecting the heart and kidneys. So, for the total life expectancy of people with alkaptonuria, the prognosis is favorable.
But the quality of life is reduced due to intense pain in the joints or spine with significant limitation of mobility, often progressive.

