Achondroplasia
Last reviewed: 23.04.2024

All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.
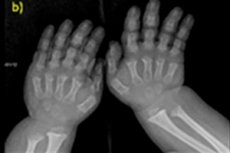
There are many rare diseases of a congenital nature, and one of them is a violation of bone growth - achondroplasia, which leads to a pronounced disproportionate short stature.
In the section on developmental anomalies of ICD-10, the code for this type of hereditary osteochondral dysplasia with defects in the growth of tubular bones and spine is Q77.4 [1]
Epidemiology
The statistics of various studies regarding the prevalence of achondroplasia are ambiguous. Some argue that this anomaly occurs in one newborn out of 10 thousand, others - in one of 26-28 thousand, and still others - 4-15 cases per 100 thousand. [2]
There is also information that when the father is over 50 years old, the incidence of achondroplasia in children is one case in 1875 newborns.
Causes achondroplasias
The cause of achondroplasia lies in the violation of osteogenesis, in particular, one of the types of intrauterine ossification of the diaphysis of the tubular bones of the skeleton - intracartilaginous (endochondral) ossification, during which there is a modification of cartilage into bone tissue. For details see - Development and growth of bones
Violation of ossification of long bones, that is, fetal achondroplasia occurs due to mutations in the gene of membrane tyrosine kinases - the fibroblast growth factor receptor 3 (FGFR3 on chromosome 4p16.3), which affects the growth and differentiation of cells. The presence of FGFR3 mutations is associated with genetic instability and changes in the number of chromosomes (aneuploidy).
The child receives achondroplasia as an autosomal dominant trait, that is, he receives one copy of the mutant gene (which is dominant) and one normal gene on a pair of non-sex (autosomal) chromosomes. Thus, the type of inheritance of this defect is autosomal dominant, and the anomaly can manifest itself in 50% of offspring when the combination of alleles of a given gene (genotype) is crossed.
In addition, mutations can be sporadic, and, as practice shows, in 80% of cases, children with achondroplasia are born to parents of normal height.
Risk factors
The main risk factors for the birth of children with achondroplasia are hereditary. If one of the parents has this defect, then the probability of having a sick child is estimated at 50%; in the presence of this anomaly in both parents - also 50%, but with a 25% risk of homozygous achondroplasia, leading to death before birth or in early infancy.
With the age of the father (closer to 40 years and older), the risk of a new mutation (de novo mutation) of the FGFR3 gene increases.
Pathogenesis
Explaining the pathogenesis of achondroplasia, experts emphasize the importance of the transmembrane protein tyrosine protein kinase (encoded by the FGFR3 gene) in the regulation of division, differentiation and apoptosis of cells in the cartilaginous tissue of growth plates - chondrocytes, as well as the normal development of the skeleton - osteogenesis and bone mineralization.
During embryonic development, in the presence of a gene mutation, fibroblast growth factor 3 receptors become more active. Strengthening their functions disrupts the transmission of cellular signals and the interaction of the extracellular part of this protein with polypeptide fibroblast growth factors (FGF). As a result, a failure occurs: the stage of proliferation of cartilage tissue cells becomes shorter, and their differentiation begins earlier than the prescribed time. All this leads to improper formation and fusion of the bones of the skull and skeletal dysplasia - a decrease in long bones, which is accompanied by pronounced short stature or dwarfism.
And two-thirds of cases of dwarfism are associated precisely with achondroplasia.
Symptoms achondroplasias
Abnormal bone growth causes such clinical symptoms of achondroplasia as:
- pronounced short stature (disproportionate dwarfism) with an average adult height of 123-134 cm;
- shortening of the proximal lower and upper limbs with a relatively normal torso size;
- shortened fingers and toes;
- enlarged head (macro or megalocephaly); [3]
- specific facial features in the form of a protruding forehead and hypoplasia of the middle part of the face - a depressed bridge of the nose.
- narrow craniocervical junction. Some babies with achondroplasia die in the first year of life from complications associated with the craniocervical junction; Population studies show that this excess risk of death can be as high as 7.5% without assessment and intervention. [4]
- Middle ear dysfunction is often a problem [5]and, if not properly treated, can result in conductive hearing loss of sufficient severity to interfere with speech development. More than half of the children will need a pressure equalization tube. [6] Overall, about 40% of people with achondroplasia have functionally significant hearing loss. Expressive language development is also often delayed, although the strength of the association between hearing loss and expressive speech problems is questionable.
- curvature of the legs is very common in patients with achondroplasia. More than 90% of untreated adults have some degree of bowing. [7] Worship is actually a complex deformity that results from a combination of lateral tilt, internal tibial torsion, and dynamic knee instability. [8]
Infants with achondroplasia are characterized by muscle hypotonia, due to which they later begin to learn movement and walking skills. Intelligence and cognitive abilities are not affected by this developmental disability. [9], [10]
Consequences and complications
For this type of hereditary osteochondral dysplasia, the following complications and consequences are characteristic:
- recurrent ear infections;
- obstructive sleep apnea;
- hydrocephalus;
- malocclusion and twisted teeth:
- deformity of the legs (varus or valgus) with a change in gait;
- hypertrophied lordosis of the lumbar spine or its curvature (thoracolumbar kyphosis or lumbar scoliosis) - with back pain when walking;
- joint pain (due to improper placement of bones or compression of nerve roots);
- spinal stenosis and spinal cord compression; The most common medical complaint in adulthood is symptomatic spinal stenosis involving L1-L4. Symptoms range from intermittent, reversible exercise-induced claudication to severe, irreversible impairment of leg function and urinary continence. [11] Lameness and stenosis can cause both sensory (numbness, pain, feeling of heaviness) and movement symptoms (weakness, stumbling, limited endurance when walking). Vascular lameness results from the swelling of blood vessels after standing and walking and is completely reversible with rest. Spinal stenosis is an actual lesion of the spinal cord or nerve root by stenotic bone of the spinal canal, and the symptoms are irreversible. Symptoms localized to a specific dermatome can result from stenosis of specific nerve root openings.
- reduction of the thoracic region with restriction of the growth of the lungs and a decrease in their function (in the form of severe shortness of breath). During infancy, a small group of people with achondroplasia have restrictive lung problems. Small breasts and increased chest compliance combine to result in decreased lung volume and restrictive lung disease [12]
Other orthopedic problems
- Weakness of the joints. Most joints are hypermobile during childhood. In general, this has little impact, with the exception of knee instability in some people.
- Discoid lateral meniscus. This newly identified structural abnormality can lead to chronic knee pain in some people. [13]
- Arthritis. Constitutive activation of FGFR-3, as in achondroplasia, may protect against the development of arthritis. [14]
- Acanthosis nigricans occurs in about 10% of people with achondroplasia. [15]In this population, this does not reflect hyperinsulinemia or malignant neoplasm.
Homozygous achondroplasia, caused by biallelic pathogenic variants of nucleotide 1138 of FGFR3, is a serious disease with radiological changes that are qualitatively different from those of achondroplasia. Early death occurs as a result of respiratory failure due to a small chest and neurological deficits due to cervicomedullary stenosis [Hall 1988].
Diagnostics achondroplasias
In most patients, achondroplasia is diagnosed based on characteristic clinical signs and radiographic findings. In infants or in the absence of certain symptoms, genetic testing - a karyotype test - is used to make an accurate diagnosis . [16]
When performing prenatal diagnostics using molecular genetics, tests may be done on the amniotic (amniotic) fluid or chorionic villus sample.
Signs of achondroplasia on fetal ultrasound - shortening of the limbs and typical facial features - are visualized after 22 weeks of pregnancy.
Instrumental diagnostics also includes an X-ray of the skeleton or ultrasound of bones . And radiography confirms the diagnosis on data such as a large skull with a narrow occipital foramen and a relatively small base; short tubular bones and shortened ribs; short and flattened vertebral bodies; narrowed spinal canal, reduced size of the iliac wings.
Differential diagnosis
Differential diagnosis with pituitary dwarfism (dwarfism) , congenital spondyloepiphyseal and diastrophic dysplasia, hypochondroplasia, Shereshevsky-Turner and Noonan syndromes, pseudoachondroplasia is required. So, the difference between pseudoachondroplasia and achondroplasia is that in patients with dwarfism with pseudoachondroplasia, the size of the head and facial features are normal.
Who to contact?
Treatment achondroplasias
Recommendations for monitoring the health of children with achondroplasia have been set out by the Genetics Committee of the American Academy of Pediatrics. These recommendations serve as a guide and do not replace individual decision making. A recent review [Pauli & Botto 2020] also includes guidance from a guide. There are specialized clinics for the treatment of skeletal dysplasia; their recommendations may differ slightly from these general recommendations.
Recommendations include (but are not limited to) the following.
Hydrocephalus. If signs or symptoms of increased intracranial pressure appear (for example, accelerated head growth, a constantly bulging fontanelle, a noticeable increase in the protrusion of superficial veins on the face, irritability, vomiting, changes in vision, headache), a referral to a neurosurgeon is necessary.
The assumed etiology of hydrocephalus in achondroplasia is increased intracranial venous pressure due to stenosis of the jugular foramen. Thus, ventriculoperitoneal shunting was the standard treatment. However, endoscopic third ventriculostomy may be beneficial in some people, [17]implying that other mechanisms, such as blockage of the ventricular outlets due to craniocervical stenosis, may be of value. [18]
Narrowing of the craniocervical junction. The best predictors of the need for suboccipital decompression are:
- Hyperreflexia or clonus of the lower extremities
- Central hypopnea on polysomnography
- Reduction in the size of the foramen magnum, determined by computed tomography of the craniocervical junction and compared with the norms for children with achondroplasia. [19]
- Signs of spinal cord compression and / or T 2 weighted signal abnormalities; has recently been suggested as another factor that should be considered when deciding to work.
If there are clear signs of symptomatic compression, you should urgently contact a pediatric neurosurgeon for decompression surgery. [20]
Obstructive sleep apnea. Treatment may include the following:
- Adenotonsillectomy
- Positive airway pressure
- Tracheostomy in extreme cases
- Reducing weight
These interventions can lead to improved sleep disturbances and some improvement in neurological function. [21]
In the rare cases where the obstruction is severe enough to warrant a tracheostomy, surgery to advance the midface has been used to relieve upper airway obstruction. [22]
Dysfunction of the middle ear. If necessary, aggressive treatment of frequent middle ear infections, persistent fluid in the middle ear, and subsequent hearing loss should be undertaken. Long-acting tubes are recommended because they are often needed up to seven or eight years of age. [23]
If problems arise at any age, it is recommended to apply appropriate treatment methods.
Low stature. A number of studies have evaluated growth hormone (GH) therapy as a possible treatment for short achondroplasia. [24]
In general, these and other series show an initial acceleration of growth, but its effect decreases over time.
On average, you can expect an increase in the height of an adult by only about 3 cm.
Extended limb lengthening using various techniques remains an option for some. You can increase your height up to 30-35 cm. [25] Complications are frequent and can be serious.
While some advocate performing these procedures as early as six to eight years of age, many pediatricians, clinical geneticists, and ethicists advocate postponing such surgery until the young person can participate in an informed decision.
In North America at least, only a small proportion of affected individuals choose to undergo extended limb lengthening. The Little People of America Medical Advisory Board has released a statement regarding the use of extended limb lengthening.
Obesity. Measures to prevent obesity should start early in childhood. Standard treatments for obesity should be effective in people with achondroplasia, although the calorie requirements are lower. [26]
Standard weight and weight-for-height grids specific for achondroplasia should be used to track progress. It is important to note that these curves are not ideal weight-for-height curves; they were obtained from thousands of data points from people with achondroplasia.
Body mass index (BMI) standards have been developed for children aged 16 and under. [27]BMI is not standardized for adults with achondroplasia; comparison with BMI curves for average height will give incorrect results. [28]
Deformation of the varus. Annual orthopedic follow-up is recommended by either a provider familiar with achondroplasia or an orthopedic surgeon. Surgical criteria published. [29]
The presence of progressive symptomatic bending requires referral to a podiatrist. The deformity of the varus itself without symptoms usually does not require surgical correction. Various interventions can be selected (eg, guided growth using eight plates, valgus osteotomy and derotation osteotomy). There are no controlled studies comparing the outcome of treatment options.
Kyphosis. Infants with achondroplasia often develop flexible kyphosis. A protocol is available to help prevent the development of fixed angular kyphosis, which includes avoiding flex-back strollers, swings, and carrying. Council against sitting without support; always apply back pressure when holding your baby.
- Kyphosis improves or goes away in most children after orthograde and walking. [30]
- In children who do not have spontaneous remission after increasing torso strength and starting to walk, fixation is usually sufficient to prevent thoracolumbar kyphosis from persisting. [31]
- If severe kyphosis persists, spinal surgery may be required to prevent neurological complications. [32]
Spinal stenosis. If serious signs and / or symptoms of spinal stenosis develop, an urgent referral to a surgical specialist is needed.
Wide and wide laminectomy is usually recommended. The relevance of the procedure depends on the level (eg, thoracic or lumbar) and the degree of stenosis. Patients had better outcomes and improved function than they had previously undergone surgery after onset of symptoms [33]
Immunization. Nothing about achondroplasia rules out all routine vaccinations. Given the increased respiratory risk, DTaP, pneumococcal and influenza vaccines are especially important.
Adaptive needs. Due to the small growth, environmental changes are necessary. In school, this may include stools, lowered light switches, toilets of appropriate heights or other means of access, lower desks, and leg rests in front of chairs. All children should be able to leave the building on their own in the event of an emergency. Small hands and weak ligaments can make fine motor skills difficult. Appropriate adaptations include the use of smaller keyboards, heavier nibs, and smoother writing surfaces. Most children must have an IEP or 504.
Pedal extensions are almost always needed for riding. Workspace modifications such as lower desks, smaller keyboards, steps and toilet access may also be required.
Socialization. Due to the very noticeable short stature associated with achondroplasia, sick people and their families may find it difficult to socialize and adapt to school.
Support groups such as Little People of America, Inc. (LPA) can help families address these issues through peer support, example, and social awareness programs.
Information on employment, education, the rights of persons with disabilities, adoption of short children, medical problems, appropriate clothing, adaptive accommodations and parenting is available through the national newsletter, seminars and workshops.
There are no medications, as well as non-medicinal agents, that can cure this congenital malformation.
The most commonly used physiotherapy treatment; may also require treatment for hydrocephalus (bypass grafting or endoscopic ventriculostomy), obesity, [34]sleep apnea, [35]middle ear infection, or spinal stenosis.
In some clinics, after the child is five to seven years old, they undertake surgical treatment: lengthening the bones of the lower leg, thighs and even the humerus or correcting deformities - using operations and special orthopedic devices - in three to four stages, with a duration of each up to 6-12 months...
Research phase therapy
The introduction of a C-type natriuretic peptide analogue is undergoing clinical trials. Early results showed that it was well tolerated and resulted in an increase in growth rate from baseline in children with achondroplasia ( trial site ). [36]The conjugated C-type natriuretic peptide is also currently in clinical trials (trial сайт испытанийsite ) [37]Other considerations include tyrosine kinase inhibition [38], meclizine [39]and soluble recombinant human FGFR3 decoy. [40]
Search clinicaltrials.gov in the US and EU Clinical Trials Registry in Europe for information on clinical trials for a wide range of diseases and conditions.
Prevention
The only preventive measure is prenatal diagnosis of congenital diseases . [41], [42]
Forecast
How long do people with achondroplasia live? Average life expectancy is about 10 years less.
Since pathological changes in bone tissue and in joints lead to limitation of self-care and movement, children with this diagnosis receive the status of disabled people. In the long term, most patients have a normal prognosis, but with age there is an increased risk of heart disease. [43]

