Medical expert of the article
New publications
Goodpasture's syndrome: causes, symptoms, diagnosis, treatment
Last reviewed: 12.07.2025

All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.
Goodpasture syndrome is an autoimmune syndrome involving alveolar pulmonary hemorrhage and glomerulonephritis caused by circulating anti-GBM antibodies. Goodpasture syndrome most often develops in individuals with a genetic predisposition who smoke cigarettes, but hydrocarbon inhalation and viral respiratory tract infections are possible additional factors. Symptoms of Goodpasture syndrome include dyspnea, cough, fatigue, hemoptysis, and/or hematuria. Goodpasture syndrome is suspected in patients with hemoptysis or hematuria and is confirmed by the presence of anti-GBM antibodies in the blood. Treatment of Goodpasture syndrome includes plasma exchange, glucocorticoids, and immunosuppressants such as cyclophosphamide. Prognosis is good if treatment is started before respiratory or renal failure develops.
Goodpasture's syndrome was first described by Goodpasture in 1919. Goodpasture's syndrome is a combination of glomerulonephritis and alveolar hemorrhage in the presence of anti-GBM antibodies. Goodpasture's syndrome most often presents as a combination of diffuse alveolar hemorrhage and glomerulonephritis, but sometimes causes isolated glomerulonephritis (10-20%) or lung involvement (10%). Men are affected more often than women.
What causes Goodpasture syndrome?
The cause of the disease has not been precisely determined. A genetic predisposition to Goodpasture's syndrome is assumed, its marker is considered to be the presence of HLA-DRW2. There is a point of view on the possible role of a previous viral infection (hepatitis A virus and other viral diseases), industrial hazards, and medications (primarily D-penicillamine).
The basis of the pathogenesis of Goodpasture's syndrome is the formation of autoantibodies to the basement membranes of the glomerular capillaries of the kidneys and alveoli. These antibodies belong to the IgG class, they bind to the antibodies of the basement membranes in the presence of the C3 component of the complement with the subsequent development of immune inflammation of the kidneys and alveoli of the lungs.
Anti-GBM antibodies are directed against the noncollagenous (NC-1) domain of the 3 chain of type IV collagen, which is found in highest concentration in the basement membranes of renal and pulmonary capillaries. Exposure to environmental factors - smoking, viral ARIs, and inhalation of hydrocarbonate suspensions (more often) - and, less often, pneumonia activates the presentation of alveolar capillary antigens to circulating antibodies in people with a hereditary predisposition (most often these are carriers of the HLA-DRwl5, -DR4, and -DRB1 alleles). Circulating anti-GBM antibodies bind to basement membranes, fix complement, and induce a cellular inflammatory response, leading to the development of glomerulonephritis and/or pulmonary capillaritis.
There is probably a certain commonality of autoantigens of the basal membrane of the glomerular capillaries of the kidneys and alveoli. The autoantigen is formed under the influence of the damaging effect of the etiologic factor. An unknown etiologic factor damages and modifies the structure of the basal membranes of the kidneys and lungs. Excretion of the resulting degradation products of the glomerular basal membranes of the kidneys slows down and decreases when they are damaged, which naturally creates the prerequisites for the development of autoimmune damage to the kidneys and lungs. It is still not completely known which component of the basal membrane becomes the autoantigen. At present, it is assumed that this is the internal structural component of the glomerular basal membrane of the kidney, the a3-chain of type 4 collagen.
The formed immune complexes are deposited along the basal membranes of the glomerular capillaries, which leads to the development of an immune inflammatory process in the renal glomerulus (glomerulonephritis) and alveoli (alveolitis). The main cells involved in the development of this immune inflammation are T-lymphocytes, monocytes, endotheliocytes, polymorphonuclear leukocytes, alveolar macrophages. The interaction between them is provided by molecular mediators, cytokines (growth factors - platelet, insulin-like, b-transforming; interleukin-1, tumor necrosis factor, etc.). Arachidonic acid metabolites, free oxygen radicals, proteolytic enzymes, adhesive molecules play a major role in the development of immune inflammation.
Activation of alveolar macrophages is of great importance in the development of alveolitis in Goodpasture's syndrome. In the activated state, they secrete about 40 cytokines. Group I cytokines (chemotaxins, leukotrienes, interleukin-8) enhance the flow of polymorphonuclear leukocytes into the lungs. Group II cytokines (growth factors - platelet, macrophage) promote the movement of fibroblasts into the lungs. Alveolar macrophages also produce active forms of oxygen, proteases, which damage lung tissue.
Pathomorphology of Goodpasture's syndrome
The main pathomorphological manifestations of Goodpasture syndrome are:
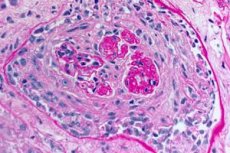
- predominant damage to the microcirculatory bed of the kidneys and lungs. In the lungs, a picture of venulitis, arteriolitis, capillaritis with pronounced phenomena of destruction and proliferation is observed; damage to the capillaries is observed mainly in the interalveolar septa, alveolitis with hemorrhagic exudate in the alveoli develops. Kidney damage is characterized by the development of extracapillary proliferative glomerulonephritis with subsequent formation of hyalinosis and fibrosis, which leads to the development of renal failure;
- pronounced intraalveolar hemorrhages;
- development of pulmonary hemosiderosis and pneumosclerosis of varying degrees of severity, as a result of the evolution of alveolitis.
Symptoms of Goodpasture's syndrome
The disease most often presents with clinical manifestations of pulmonary pathology. Hemoptysis is the most prominent symptom; however, hemoptysis may be absent in the presence of hemorrhagic manifestations, and the patient may present with only infiltrative changes on chest radiography or with an infiltrate and respiratory distress and/or failure. Dyspnea (primarily with exertion), cough, malaise, decreased ability to work, chest pain, fever, and weight loss are common. Up to 40% of patients have macrohematuria, although pulmonary hemorrhage may precede renal manifestations by weeks to years.
During hemoptysis, shortness of breath may increase. Weakness and decreased ability to work are also a concern.
Symptoms of Goodpasture's syndrome vary over time, ranging from clear lungs on auscultation to crackling and dry rales. Some patients have peripheral edema and pallor due to anemia.
During examination, attention is drawn to pale skin, cyanosis of the mucous membranes, pastosity or pronounced swelling of the face, decreased muscle strength, and weight loss. Body temperature is usually elevated to febrile levels.
When percussing the lungs, a shortening of the percussion sound may be determined over extensive foci of pulmonary hemorrhage, but this is observed rarely; more often, there are no changes in the percussion sound.
A characteristic auscultatory sign of Goodpasture's syndrome is dry and wet wheezing, the number of which increases significantly during or after hemoptysis.
When examining the cardiovascular system, arterial hypertension is revealed, possibly an increase in the border of relative cardiac dullness to the left, muffled heart sounds, a soft systolic murmur, and pericardial friction murmur appears with the development of severe renal failure. With progressive kidney damage against the background of significant arterial hypertension, acute left ventricular failure with a picture of cardiac asthma and pulmonary edema may develop. Usually, this situation develops in the terminal stage of the disease.
As a rule, kidney damage manifests itself later, after a certain time after the development of pulmonary symptoms. Characteristic clinical signs of renal pathology are hematuria (sometimes macrohematuria), rapidly progressing renal failure, oliguria, arterial hypertension.
In 10-15% of cases, Goodpasture's syndrome begins with clinical signs of renal pathology - the clinical picture of glomerulonephritis appears (oliguria, edema, arterial hypertension, pronounced pallor), and then symptoms of lung damage join in. Many patients may have myalgia, arthralgia.
Regardless of the onset variants, Goodpasture syndrome in most cases is severe, the disease steadily progresses, severe pulmonary and renal failure develops. The life expectancy of patients from the onset of the disease ranges from several months to 1-3 years. Most often, patients die from uremia or pulmonary hemorrhage.
What's bothering you?
Diagnosis of Goodpasture syndrome
The diagnosis of Goodpasture syndrome requires detection of serum anti-GBM antibodies by indirect immunofluorescence or, when available, by direct enzyme-linked immunosorbent assay (ELISA) with recombinant human NC-1 a3. Other serologic tests, such as antinuclear antibody (ANA) testing, are used to detect SLE and antistreptolysin-O titer to detect poststreptococcal glomerulonephritis, which may be the cause of many cases of pulmonary-renal syndrome. ANCA is positive (in peripheral specimens) in 25% of cases of Goodpasture syndrome. Renal biopsy may be indicated if glomerulonephritis is present (hematuria, proteinuria, red blood cell sludge on urinalysis, and/or renal failure). Rapidly progressive focal segmental necrotizing glomerulonephritis with a progressive course is found on biopsy in Goodpasture syndrome and all other causes of pulmonary-renal syndrome. Immunofluorescence staining of renal or lung tissue classically reveals linear deposition of IgG along glomerular or alveolar capillaries. It is also seen in diabetic kidney and fibrillary glomerulonephritis, a rare disorder causing pulmonary-renal syndrome, but GBM antibody detection in these disorders is nonspecific.
Pulmonary function tests and bronchoalveolar lavage are not diagnostic of Goodpasture syndrome but may be used to confirm the presence of diffuse alveolar hemorrhage in patients with glomerulonephritis and pulmonary infiltrates but without hemoptysis. Lavage fluid that remains hemorrhagic after multiple washes may confirm diffuse hemorrhagic syndrome, especially if there is a concomitant decrease in hematocrit.
 [ 3 ]
[ 3 ]
Laboratory diagnostics of Goodpasture syndrome
- General blood analysis. Characteristic features are iron deficiency hypochromic anemia, hypochromia, anisocytosis, poikilocytosis of erythrocytes. Leukocytosis, left shift of the leukocyte formula, and a significant increase in ESR are also observed.
- General urine analysis. Protein (the degree of proteinuria can be significant), cylinders (granular, hyaline, erythrocyte), erythrocytes (macrohematuria may occur) are found in the urine. As chronic renal failure progresses, the relative density of urine decreases, and isohyposthenuria develops in the Zimnitsky test.
- Biochemical blood test. Increased blood levels of urea, creatinine, haptoglobin, seromucoid, a2 and gamma globulins, decreased iron content.
- Immunological studies. A decrease in the number of T-lymphocyte suppressors may be detected, circulating immune complexes are detected. Antibodies to the basement membrane of the glomerular and alveolar capillaries are detected by indirect immunofluorescence or radioimmunological methods.
- Sputum analysis. Sputum contains many erythrocytes, hemosiderin, siderophages are detected.
Instrumental diagnostics of Goodpasture syndrome
- X-ray examination of the lungs. Characteristic X-ray signs are pulmonary infiltrates in the root region spreading to the lower and middle parts of the lungs, as well as progressive, symmetrical, bilateral cloud-like infiltrates.
- Study of the function of external respiration. Spirometry reveals a restrictive type of respiratory failure (decreased vital capacity), as the disease progresses, an obstructive type of respiratory failure joins in (decreased FEV1, Tiffeneau index).
- ECG. Signs of severe myocardial dystrophy of anemic and hypoxic genesis are revealed (reduction in the amplitude of T waves and the ST interval in many leads, most often in the left chest leads). With severe arterial hypertension, signs of left ventricular myocardial hypertrophy appear.
- Blood gas analysis reveals arterial hypoxemia.
- Examination of lung and kidney biopsies. A biopsy of the lung tissue (open biopsy) and kidneys is performed for final verification of the diagnosis if it is impossible to accurately diagnose the disease using non-invasive methods. Histological and immunological examination of the biopsies is performed. The following signs are characteristic of Goodpasture's syndrome:
- the presence of morphological signs of glomerulonephritis (most often extracapillary), hemorrhagic alveolitis, hemosiderosis and interstitial fibrosis;
- detection of linear deposits of IgG and complement component C3 on the basement membranes of the pulmonary alveoli and renal glomeruli using the immunofluorescence method.
Diagnostic criteria for Goodpasture syndrome
When making a diagnosis of Goodpasture syndrome, it is advisable to use the following criteria.
- A combination of pulmonary pathology and kidney pathology, i.e. hemoptysis (often pulmonary hemorrhage), shortness of breath and symptoms of glomerulonephritis.
- Steadily progressive course of the disease with the development of respiratory and renal failure.
- Development of iron deficiency anemia.
- Detection during radiographic examination of the lungs of multiple bilateral cloud-like infiltrates against the background of reticular deformation of the pulmonary pattern.
- Detection in the blood of high titers of circulating antibodies to the basement membrane of the renal glomeruli and alveoli.
- Detection of linear deposits of IgG and complement component C3 on the basement membranes of glomerular and alveolar capillaries.
- Absence of other systemic (except pulmonary and renal) manifestations.
Differential diagnosis of Goodpasture syndrome
Goodpasture's syndrome must be differentiated from a number of diseases manifested by hemoptysis or pulmonary hemorrhage. It is necessary to exclude oncological diseases of the bronchi and lungs, tuberculosis, lung abscesses, bronchiectasis, heart and vascular diseases (leading to congestion and hypertension in the pulmonary circulation), systemic vasculitis, hemorrhagic diathesis.
Goodpasture Syndrome Screening Program
- General blood and urine tests.
- Biochemical blood test: determination of total protein and protein fractions, creatinine and urea, transaminases, seromucoid, haptoglobin, fibrin, iron.
- Sputum analysis: cytological examination, determination of siderophages.
- Immunological studies: determination of the content of B- and T-lymphocytes, subpopulations of T-lymphocytes, immunoglobulins, circulating immune complexes, antibodies to the basement membranes of the glomeruli of the kidneys and alveoli.
- X-ray examination of the lungs.
- ECG.
- Spirometry.
- Examination of lung and kidney biopsies.
What tests are needed?
Treatment of Goodpasture's syndrome
Treatment of Goodpasture syndrome includes daily or every other day plasma exchange for 2 to 3 weeks (4 L plasma exchange) to remove anti-GBM antibodies, combined with intravenous glucocorticoids (usually methylprednisolone 1 g over at least 20 minutes every other day 3 times with prednisolone 1 mg/kg body weight daily) and cyclophosphamide (2 mg/kg once daily) for 6 to 12 months to prevent formation of new antibodies. Therapy may be tapered when pulmonary and renal function cease to improve. Long-term mortality is related to the degree of renal impairment at disease onset; patients requiring dialysis early and those with more than 50% crescentic nephrons on biopsy have survival times of less than 2 years and often require dialysis unless renal transplantation is considered. Hemoptysis may be a good prognostic sign because it leads to earlier detection of the disease; the minority of patients who are ANCA-positive respond better to treatment for Goodpasture syndrome. Recurrence occurs in a small percentage of cases and is associated with continued smoking and respiratory tract infection. In patients with end-stage renal disease who have had a kidney transplant, the disease may recur in the graft.
What is the prognosis for Goodpasture syndrome?
Goodpasture syndrome is often rapidly progressive and can be fatal unless promptly diagnosed and treated; the prognosis is good when treatment is started before respiratory or renal failure develops.
Immediate survival at the time of pulmonary hemorrhage and respiratory failure is associated with ensuring airway patency; endotracheal intubation and mechanical ventilation are recommended for patients with borderline arterial blood gas levels and impending respiratory failure.

