Medical expert of the article
New publications
Gastroesophageal reflux disease and pregnancy
Last reviewed: 12.07.2025

All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.
Gastroesophageal reflux disease (GERD) is a chronic recurrent disease caused by a disorder of the motor-evacuation function of the organs of the gastroesophageal zone and characterized by spontaneous or regularly recurring reflux of gastric or duodenal contents into the esophagus, which leads to damage to the distal esophagus with the development of erosive-ulcerative, catarrhal and/or functional disorders.
Epidemiology
Heartburn, the main symptom of gastroesophageal reflux disease, occurs in approximately 50% of pregnant women, reaching 80% according to some studies. [ 1 ] About 25% of pregnant women experience heartburn on a daily basis. [ 2 ] Heartburn is such a common problem for pregnant women that both the patients themselves and many obstetricians consider it a normal manifestation of pregnancy that does not require special attention.
Approximately 17% of pregnant women experience heartburn and regurgitation simultaneously.[ 3 ] Recently, the incidence of reflux symptoms in the 3rd trimester has been reported to be around 25%, with the severity of heartburn steadily increasing throughout pregnancy.[ 4 ],[ 5 ]
Body mass index before pregnancy, weight gain during the last pregnancy, race do not affect the frequency of occurrence and severity of the symptom. The development of heartburn in the first pregnancy increases the risk of its recurrence in subsequent ones.
Heartburn is often a consequence of an exacerbation of previously existing GERD. Our experience shows that out of 55 pregnant women with reflux esophagitis confirmed endoscopically, only 10 (18.2%) developed the disease for the first time in their lives during pregnancy. Another point of view is that most women begin to complain of heartburn only when it actually worsens their quality of life and causes significant anxiety, i.e. much later than it actually appears.
Causes GERD in pregnancy
GERD during pregnancy is likely caused by decreased pressure in the lower esophageal sphincter due to increased maternal estrogen and progesterone levels during pregnancy. Hormonal changes during pregnancy may also decrease gastric motility, leading to increased gastric emptying time and an increased risk of GERD.
Pathogenesis
The occurrence of GERD during pregnancy is multifactorial, involving both hormonal and mechanical factors. It is often the result of a progressive decrease in lower esophageal sphincter pressure due to a gradual increase in circulating estrogen and progesterone.[ 8 ] The lowest lower esophageal sphincter pressure occurs at 36 weeks of gestation.[ 9 ] Other factors that may also play a role in GERD include increased intragastric pressure due to an enlarging uterus and changes in gastrointestinal motility due to ineffective esophageal motility with prolonged emptying times.[ 10 ]
Symptoms GERD in pregnancy
The symptoms of gastroesophageal reflux disease during pregnancy are almost the same as those outside of it. The main symptom is heartburn, which usually develops after eating, especially after eating large, fatty, fried and spicy foods, manifested by a burning sensation in the chest area and / or regurgitation. [ 11 ] Some women, in order to avoid heartburn, prefer to eat once a day, which can lead to significant weight loss. Heartburn lasts from several minutes to hours, repeats many times a day, intensifying in a horizontal position, when turning from one side to the other. Some pregnant women pay attention to the fact that heartburn bothers more on the left side. In addition, bending the body forward, for example, to put on or fasten shoes (the "lace" symptom), provokes its appearance.
In some cases, to relieve heartburn that occurs at night during sleep, the patient is forced to get up, walk around the room for a while, and drink some water. Some women have to sleep sitting in a chair. The feeling of heartburn is accompanied by a painful feeling of melancholy and a depressed mood. Against the background of prolonged heartburn, pain behind the breastbone, odynophagia, and belching of air may occur. Often the pain radiates to the back of the head, the interscapular space, and intensifies during or immediately after eating. Sometimes, patients with heartburn experience increased salivation.
Thus, during pregnancy, the primary diagnosis of gastroesophageal reflux disease should be based on the clinical manifestations of the disease, since the sensitivity and specificity of such a symptom as heartburn, which appears after eating or when the patient is lying on her back, reaches 90%.
Physical examination may reveal moderate tenderness to palpation in the epigastric region.
Exacerbation of GERD (gastroesophageal reflux disease), reflux esophagitis are more often observed in the second half of pregnancy. In the first trimester, heartburn and exacerbation of GERD are often provoked by early toxicosis - vomiting of pregnant women. Therefore, if vomiting occurs at the end of the gestation period (the last 6-7 weeks), this symptom should not be ignored, since vomiting may be a sign of a hernia of the esophageal opening of the diaphragm or developing complications.
What's bothering you?
Forms
In 2002, at the World Congress of Gastroenterologists in Los Angeles, a new clinical classification of gastroesophageal reflux disease was adopted, according to which the following are distinguished:
- nonerosive (or endoscopically negative) form of the disease (NERD), i.e. GERD without signs of esophagitis; this definition applies to cases where a patient with manifestations of the disease, primarily heartburn, meeting the clinical criteria for gastroesophageal reflux disease, did not have damage to the esophageal mucosa;
- erosive-ulcerative (or endoscopically positive) form of the disease, including complications in the form of ulcers and esophageal strictures;
- Barrett's esophagus (metaplasia of stratified squamous epithelium into columnar epithelium in the distal esophagus as a consequence of gastroesophageal reflux disease. The isolation of this form of the disease is due to the fact that this form of metaplasia is considered a precancerous condition. To date, there are no cases of the disease in pregnant women described in the literature).
Complications and consequences
Complications of GERD during pregnancy, including ulceration, bleeding, and esophageal stricture, are rare, perhaps because the duration of esophagitis in pregnant women is relatively short.
Diagnostics GERD in pregnancy
The diagnosis of GERD during pregnancy is established on the basis of complaints, anamnesis data, and the results of instrumental examination.
X-ray examination is not used in pregnant women due to the possible damaging effect on the fetus; pH-metry can be used, but the need for its use is questionable.
Esophagogastroduodenoscopy
Esophagogastroduodenoscopy (EGDS) is the method of choice for diagnosing GERD (gastroesophageal reflux disease), especially its complications. Although the method is burdensome for the mother, its safety for the fetus, high information content, the possibility of accurate diagnosis and differential diagnosis of diseases put it in 1st place among instrumental methods for diagnosing pathology of the upper digestive tract in pregnant women. Having started using endoscopy in urgent situations, we came to the conclusion about the need to use it in routine examination of pregnant women with appropriate indications.
Indications for EGDS:
- acute esophageal-gastric bleeding;
- suspected injury or perforation of the esophagus, stomach or duodenum; suspected presence of a foreign body;
- to confirm or exclude a tumor process;
- acute attacks of abdominal pain, persistent dyspeptic complaints in combination with pain in the upper abdomen with negative results of ultrasound examination of the abdominal organs;
- suspected severe peptic esophagitis, esophageal stricture;
- in pregnant women with liver cirrhosis to exclude or confirm the presence of varicose veins of the esophagus.
Planned fibroendoscopy is contraindicated for pregnant women with deformation of the cervicothoracic spine, pronounced kyphosis, scoliosis or lordosis; esophageal stenosis, the size of which is smaller than the diameter of the endoscope; rigidity of the pharynx; large goiter; excessive vomiting of pregnant women; nephropathy, eclampsia or preeclampsia; placenta previa, high myopia. Isthmic-cervical insufficiency in combination with the threat of termination of pregnancy can be singled out as a relative contraindication.
Another safe, highly informative instrumental method for diagnosing GERD in pregnant women is ultrasound. A reliable echographic sign of a hernia is an increase in the diameter of the cross-section of the digestive tract at the level of the esophageal opening of the diaphragm by more than 1.58 +/– 0.18 cm, and ultrasound signs of gastroesophageal reflux are expansion of the abdominal esophagus within 9 minutes from the start of the echocontrast study and an increase in the diameter of the esophagus by more than 0.35 +/– 0.06 cm.
What tests are needed?
Who to contact?
Treatment GERD in pregnancy
The basis of treatment for GERD (heartburn) is the maximum strengthening of protective factors against reflux and the weakening of the aggressive acid-peptic factor, which should begin with following recommendations for lifestyle changes and diet. [ 12 ]
Lifestyle (see table ) and dietary changes should be considered as first-line treatment during pregnancy, however, if heartburn is severe enough, treatment should be started after consultation with a physician (recommendation level C). [ 13 ], [ 14 ]
A woman should avoid positions that promote heartburn. If there are no contraindications, sleep with the head of the bed elevated (it should be raised at an angle of 15°, “high” pillows alone are not enough). [ 15 ] It is extremely undesirable to stay in an inclined position for a long time, be forced to lie in bed with the head of the bed lowered, perform gymnastic exercises associated with abdominal tension, wear tight belts, corsets. [ 16 ] It is necessary to avoid constipation, if it develops, since any straining leads to an increase in intra-abdominal pressure, the release of acidic gastric contents into the esophagus and the appearance of heartburn.
After eating, you should not lie down - it is better to sit or even stand: this promotes faster evacuation of food from the stomach.
Fractional meals (5-7 times a day) in small portions are recommended; a woman should avoid overeating. It is advisable to include alkaline reaction foods in the diet ("food antacids"): milk, cream, sour cream, cottage cheese, steamed protein omelets, boiled meat, fish, poultry, butter and vegetable oil, white bread. Vegetable dishes and side dishes should be boiled or mashed. It is better to bake apples. It is not recommended to eat fatty fried meat, poultry, fish, smoked foods, hot sauces and seasonings, sour fruit juices and compotes, vegetables containing coarse fiber (white cabbage, radish, horseradish, onions, garlic), mushrooms, black bread, chocolate, carbonated and fizzy drinks, hot tea, black coffee. [ 17 ]
In case of minor heartburn, these measures may be quite sufficient. In cases of severe heartburn, the appearance of other symptoms of GERD (gastroesophageal reflux disease), it is necessary to discuss with the patient all the positive and possible negative aspects of drug therapy.
Drug treatment of GERD during pregnancy
Various pharmacologic interventions are available to control symptoms, but the potential risks to the patient, fetus, and newborn child should be discussed with the patient. The critical teratogenic period during pregnancy is from day 31 (in a 28-day menstrual cycle) to day 71 from the last menstrual period. Exposure to a potential teratogen before this period usually results in an all-or-nothing outcome (either fetal death or anomaly-free survival); thus, any pharmacologic agents that are not absolutely necessary should be deferred until the period of potential teratogenicity has passed. Therefore, the chosen treatment for GERD during pregnancy should minimize the potential risks. Thus, treatment options should follow a stepwise approach (Grade C recommendation). [ 18 ], [ 19 ] In this approach, the first step is lifestyle modification. If there is no response or bothersome symptoms persist, pharmacologic treatment is initiated, starting with antacids, then histamine-2 receptor antagonists (H2RAs), and finally proton pump inhibitors (PPIs) (table).[ 20 ]
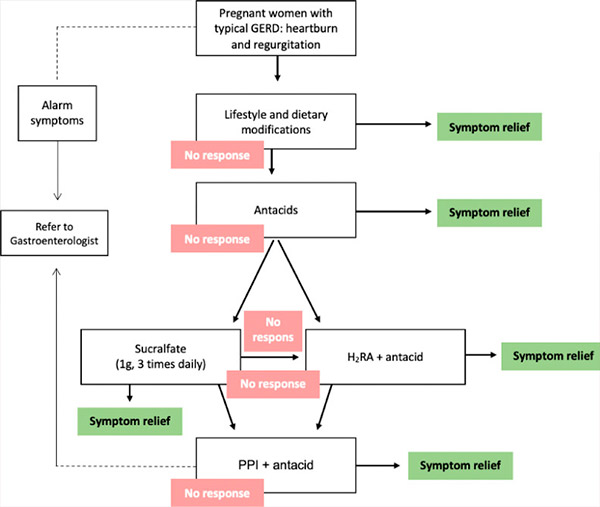
Stepwise approach to treating GERD during pregnancy. GERD = gastroesophageal reflux disease, H2RA = histamine-2 receptor antagonist, PPI = proton pump inhibitor.
Unfortunately, the drugs used to treat GERD (gastroesophageal reflux disease) have not been tested in randomized controlled trials in pregnant women. Most recommendations for their use are based on case reports and cohort studies conducted by pharmaceutical companies or on recommendations from the U.S. Food and Drug Administration (FDA).
Traditional treatments for GERD (gastroesophageal reflux disease) include antacids, sucralfate, prokinetics, H2-histamine receptor blockers, and proton pump inhibitors. The FDA has divided all medications used during pregnancy into five safety categories: A, strong, C, D, and X, based on their systemic availability and absorption, as well as reports of congenital malformations in humans and animals.
Antacids
Antacids are one of the most frequently prescribed (used) groups of medications for pregnant women, second only to iron supplements. About 30–50% of pregnant women take them to treat heartburn and other reflux symptoms.
Antacids containing aluminum, calcium, and magnesium have not been shown to be teratogenic in animal studies and are recommended as first-line treatment for heartburn and acid reflux during pregnancy.[ 21 ] High doses and prolonged use of magnesium trisilicate have been associated with nephrolithiasis, hypotension, and respiratory distress in the fetus and its use is not recommended during pregnancy. Bicarbonate-containing antacids are also not recommended due to the risk of maternal and fetal metabolic acidosis and fluid overload. There have also been case reports of milk-alkali syndrome in pregnant women taking daily doses greater than 1.4 g of elemental calcium derived from calcium carbonate.[ 22 ]
The preferred choice of antacids for the treatment of GERD during pregnancy are calcium-containing antacids at usual therapeutic doses, given the beneficial effect of this treatment in preventing hypertension and preeclampsia (recommendation level A). [ 23 ]
A systematic review found that calcium supplements are effective in preventing hypertension and preeclampsia. Consensus recommends the use of calcium-containing antacids given their limited side effects. However, excessive calcium carbonate intake may lead to milk-alkali syndrome; calcium carbonate-containing antacids are unlikely to have a significant effect on the neonate.[ 24 ] Similar to calcium-containing antacids, magnesium sulfate resulted in a 50% reduction in the risk of eclampsia and thus a reduction in maternal mortality in a randomized, placebo-controlled trial.[ 25 ]
The use of antacids containing magnesium bicarbonate or trisilicate is not recommended during pregnancy (recommendation level C).
Bicarbonate-containing antacids may cause fetal and maternal fluid overload and metabolic alkalosis. High doses and prolonged use of magnesium trisilicate have been associated with fetal respiratory distress, hypotension, and nephrolithiasis.[ 26 ]
Antacid drugs are usually divided into absorbable (systemic, soluble) and non-absorbable (non-systemic, insoluble). Absorbable drugs include magnesium oxide, calcium carbonate, sodium bicarbonate, the latter is often used in everyday life to get rid of heartburn, but it is not suitable for long-term systematic use. Firstly, despite the ability of baking soda to quickly relieve heartburn, its effect is short-term, and since carbon dioxide is formed when interacting with gastric juice, which has a pronounced juice-producing effect, new portions of hydrochloric acid are released again, and heartburn soon resumes with renewed vigor. Secondly, sodium contained in soda, absorbed in the intestine, can lead to the appearance of edema, which is extremely undesirable for pregnant women.
Non-absorbable antacids include magnesium carbonate basic, aluminum phosphate, aluminum hydroxide. They are highly effective and have few side effects, and can be prescribed to pregnant women without fear of exposing the mother and fetus to special risk. Animal studies have proven the absence of teratogenic effects of magnesium-, aluminum-, and calcium-containing antacids. Today, most of them are considered safe and are acceptable for use in average therapeutic doses by pregnant women. Moreover, there are observations showing that women who took magnesium oxide had pregnancy complications of nephropathy and eclampsia less often. However, it has been noted that magnesium sulfate can lead to delayed labor and weakness of labor, and the development of convulsions. Therefore, magnesium-containing antacids should be excluded in the last weeks of pregnancy.
Speaking about antacids, we cannot help but mention the popular medicines in our country, which include the main bismuth nitrate (Vikalin, Roter, Bismofalk) and colloidal bismuth subcitrate (De-Nol), which have not only an antacid but also a cytoprotective effect, which should not be used during pregnancy due to the lack of information about the possible adverse effects of bismuth salts on the fetus. Bismuth preparations are classified as category C by the FDA.
Drugs that have a protective effect on the mucous membrane
Sucralfate
In patients who have persistent GERD symptoms despite antacid use, sucralfate (1 g oral tablet 3 times daily) may be the next pharmacologic option (recommendation level C).[ 27 ]
Sucralfate is slowly absorbed, making it safe for use during pregnancy and breastfeeding. Animal studies have demonstrated its safety from teratogenic effects at doses 50 times higher than those used in humans, and the FDA has classified it as “Class B.”[ 28 ]
Only one prospective study has assessed the results of this treatment. More women in the sucralfate group experienced relief of heartburn and regurgitation compared with the lifestyle modification group (90% vs. 43%, P < 0.05).
H2-histamine receptor blockers
If symptoms persist with antacids alone, H2-blockers can be combined with antacids (recommendation level B). H2-blockers given in combination with antacids should be considered as third-line therapy for GERD during pregnancy.[ 29 ]
Although H2 blockers have been used less and less in recent years to treat GERD (gastroesophageal reflux disease) in the general population, they are the most commonly prescribed class of drugs used to treat heartburn in pregnant women who have not responded to lifestyle changes and antacids. All four classes of drugs (cimetidine, ranitidine, famotidine, and nizatidine) are FDA pregnancy category B drugs.
Cimetidine
It has been used in clinical practice for over 25 years. During this time, significant experience has been accumulated in its use in various groups of patients, including pregnant women. At the same time, according to the FDA classification, the drug is quite safe, since it does not increase the risk of congenital malformations. However, some experts believe that it should not be prescribed to pregnant women, since cimetidine can lead to feminization of male newborns.
Ranitidine
The drug's efficacy in pregnant women has been specifically studied. A double-blind, placebo-controlled, crossover study [10] compared the efficacy of ranitidine taken once or twice daily with placebo in pregnant women with GERD (gastroesophageal reflux disease) symptoms who had failed antacid treatment. Twenty women after 20 weeks of pregnancy were given 150 mg of ranitidine twice daily, or 150 mg once daily at night, or placebo. Twice-daily dosing was effective, and no side effects or adverse pregnancy outcomes were observed. [ 30 ]
There are also certain statistics, including materials based on the generalization of individual cases of ranitidine use at various stages of pregnancy. At the same time, no side effects of the drug were registered.
Experimental studies performed on rats and rabbits revealed no evidence of impaired fertility or fetotoxicity even when ranitidine was administered at a dose 160 times higher than that recommended for humans.
Several studies have been devoted to the safety of ranitidine use in the first trimester of pregnancy. A prospective cohort study conducted in 1996, which included 178 women taking H2 blockers (71% were prescribed ranitidine, 16% - cimetidine, 8% - famotidine and 5% - nizatidine) and 178 women from the control group who did not take any medications (of the same age, with similar indications in the anamnesis regarding alcohol consumption and smoking), proved the safety of the drugs. Thus, congenital malformations were observed in 2.1% of cases in patients taking H2 blockers versus 3% in the comparison group.
Similar data were obtained in the Swedish Medical Strength Registry Study in 1998: 6 (3.8%) cases of congenital malformations were registered among 156 newborns whose mothers took ranitidine during pregnancy. And the combined figures for Great Britain and Italy give a risk level of congenital malformations associated with taking the drug equal to 1.5.
The absence of teratogenic or toxic effects in experimental conditions and the data obtained in the clinic show that ranitidine is safe during pregnancy, even during the first trimester, and it is the only H2 blocker with proven efficacy in pregnant women.
Famotidine
There are few studies on the use of famotidine during pregnancy. Experimental studies on rats and rabbits indicate no fetotoxic or teratogenic effects. In the previously cited Michigan Medicaide study, congenital malformations were found in 2 (6.1%) of 33 newborns whose mothers took famotidine in the first trimester of pregnancy (compared to the predicted one case). However, the number of currently available observations is too small to draw any definitive conclusions.
Nizatidine
Safety data for nizatidine during pregnancy are also limited. Experimental studies do not support the presence of a possible embryo- or fetotoxic effect, and the only report in the literature concerns a successful pregnancy outcome in a woman who took nizatidine from the 14th to the 16th week of pregnancy. It should be noted that while nizatidine was initially classified by the FDA as category C, it was recently reclassified to category B.
Prokinetics
Prokinetics (metoclopramide, domperidone, cisapride) provide significant symptom relief comparable to the use of H2 blockers in mild forms of GERD (gastroesophageal reflux disease), but they are significantly less effective in healing erosive and ulcerative lesions of the esophageal mucosa. Metoclopramide is classified by the FDA as category B, and cisapride as category C. Only metoclopramide is used in pregnant women.
Metoclopramide
Metoclopramide, being a dopamine receptor blocker, increases the tone of the lower esophageal sphincter and thereby reduces gastroesophageal reflux, improves the kinetics and thereby self-cleaning of the esophagus, improves the evacuation function of the stomach. In pregnant women, the main indication for its use is nausea and vomiting of pregnancy. Experimental studies have shown the safety of its use during pregnancy in laboratory animals. No congenital malformations or toxic lesions of newborns due to the use of metoclopramide have been recorded in humans. At the same time, a Michigan Medicaide study recorded 10 cases of congenital malformations (8 of which were expected) (5.2%) in 192 newborns whose mothers took metoclopramide in the first trimester. Metoclopramide is classified by the FDA as category B.
Proton pump inhibitors
If H2 blockers in combination with antacids fail to sufficiently control symptom severity, it is recommended to use PPIs with the addition of antacids as rescue medications for breakthrough GERD (recommendation level C). [ 31 ]
Proton pump inhibitors (PPIs) are the most effective class of drugs used to treat both endoscopically negative and positive gastroesophageal reflux disease. Although PPIs are more effective than H2 blockers in treating GERD, they are not used as frequently in pregnant women. Therefore, safety data for this class of therapeutic agents during pregnancy are even more limited. The prevailing opinion is that PPIs should be used during pregnancy only in patients with endoscopically confirmed severe or complicated GERD who are not responding to H2 blockers.
Available PPI drugs include omeprazole, esomeprazole, lansoprazole, dexlansoprazole, rabeprazole, and pantoprazole. From a safety perspective, the FDA classifies omeprazole as a Class C drug due to potential fetal toxicity (based on animal studies), while other PPIs are classified as Class B.[ 32 ]
Omeprazole
Omeprazole is classified by the FDA in drug category C because, at human doses, it causes dose-dependent embryonic/fetal death in rats and rabbits without teratogenic effects.
On the other hand, there is information in the literature about the safety of omeprazole.
There are also several prospective studies confirming the safety of PPIs and, in particular, omeprazole in pregnant women.
And the generalized world experience allowed the company AstraZeneca to permit the use of the original drug omeprazole (Losec MAPS) during pregnancy, stating in the instructions for its medical use that "the results of the studies showed the absence of side effects of omeprazole on the health of pregnant women, on the fetus or newborn. Losek MAPS can be used during pregnancy."
Lansoprazole
Experimental studies conducted on pregnant rats and rabbits have shown that lansoprazole at doses 40 and 16 times, respectively, higher than those recommended for humans, does not have a negative effect on fertility and is not fetotoxic.
Data on the safety of clinical use of the drug in women during the gestational period are limited. The safest solution to the problem is to avoid using the drug during pregnancy, especially in the first trimester, but if there is a need for lansoprazole therapy or such therapy was carried out in the early stages of gestation, the risk to the fetus seems to be very small.
Rabeprazole, pantoprazole, esomeprazole
According to the information provided by the manufacturers, experimental data obtained on rats and rabbits indicate the safety of using these drugs during pregnancy. However, there is no information in the literature on the use of these drugs in humans, so it is better to avoid using rabeprazole, pantoprazole and esomeprazole in the treatment of GERD (gastroesophageal reflux disease) in pregnant women.
The use of rabeprazole during pregnancy has not been studied in humans; however, based on animal data for rabeprazole and human data for other PPIs, rabeprazole is expected to be safe for use during pregnancy.[ 33 ]
Prevention of aspiration syndrome during childbirth
Pregnant women have a high risk of gastric aspiration during labor, especially if the labor is performed under anesthesia. Mendelson's syndrome or acid aspiration syndrome is the most common cause of obstetric morbidity and mortality from anesthesia. That is why prevention of this complication is so important during labor. Summarizing the data obtained by various researchers, we can conclude that from the point of view of safety for the child, the most justified method for preventing acid aspiration syndrome during labor or surgical resolution is the administration of H2-histamine receptor blockers, in particular ranitidine. A whole set of studies, proving this fact, indicate that when prescribing the drug to women in labor, no negative effect on the frequency and strength of contractions, fetal heart rate, or Apgar score was recorded. In addition, no negative effect on the acidity of gastric juice in newborns was noted within 24 hours after birth. For the prevention of acid aspiration syndrome during labor or cesarean section, it is also acceptable to prescribe PPIs, as evidenced by the conclusions made by FDA experts.
Conclusion
Taking into account all the information presented in this chapter, the following algorithm for treating GERD (gastroesophageal reflux disease) in pregnant women can be proposed. In mild cases, dietary prescription and adherence to lifestyle recommendations may be sufficient.
If there is no effect, drug therapy should be started with the administration of antacids (1 therapeutic dose 3 times a day 1 hour after meals and the 4th time at night) or sucralfate (1 g 3 times a day).
If this therapeutic approach is ineffective, after a comprehensive discussion of the problem with the patient, including the safety profile of the recommended drugs, H2-histamine receptor blockers may be prescribed (once a day in the evening, after dinner). According to most researchers, ranitidine at a dose of 150 mg/day (once in the evening, after meals) is safe.
PPIs are reserve drugs for the treatment of severe and complicated cases of GERD (gastroesophageal reflux disease) after preliminary EGDS. Apparently, preference should be given to the original omeprazole, which has the best safety profile of all PPIs. Naturally, it is preferable not to prescribe antisecretory drugs in the first trimester of pregnancy.
Surgical treatment of GERD (gastroesophageal reflux disease) is not performed during pregnancy.
Treatment of GERD during breastfeeding
Although the main symptoms of GERD usually resolve soon after delivery, some women continue to experience reflux symptoms, particularly heartburn, in the postpartum period and require medical therapy.
It has been established that most systemic drugs used in the treatment of GERD (gastroesophageal reflux disease) are secreted into mother's milk and can adversely affect the development of the child. The safety of using drugs during lactation, as well as in pregnant women, is based on experimental data and literature on their use by nursing mothers.
Non-absorbable antacids (aluminum hydroxide, magnesium trisilicate) do not accumulate in breast milk and are therefore considered safe.
All H2 blockers are secreted into breast milk, so theoretically they can negatively affect the acidity of the gastric contents of newborns, inhibit drug metabolism, and stimulate the central nervous system. In 1994, the American Academy of Pediatrics classified ranitidine and famotidine as safe drugs for breastfeeding, with famotidine being more preferable because it has a lower ability to accumulate in breast milk. It is better not to prescribe nizatidine to women during lactation, since its effect is poorly studied.
Similarly, little is known about the secretion of PPIs into breast milk and their safety for the infant. PPIs appear to enter milk because they have a relatively low molecular weight. The only published study on the use of omeprazole during lactation suggests that it is safe for use in humans. An experimental study in rats showed that the drug resulted in a slowdown in the weight gain of rat pups. Therefore, given the limited number of observations, PPIs are not recommended for use during lactation. Women with severe GERD who require chronic antisecretory therapy should either discontinue breastfeeding and continue treatment or use drugs from other classes.
Thus, during pregnancy and lactation, for the treatment of GERD, it is better to prefer drugs whose action has been well studied for many years to new drugs. Only strict control of the doctor over the intake of drugs by pregnant women, prudent therapy will reduce the risk of possible undesirable effects to a minimum.
Prevention
Consists of following general “regime” and dietary measures developed for patients suffering from GERD.

