Medical expert of the article
New publications
Synthesis, secretion and metabolism of catecholamines
Last reviewed: 06.07.2025

All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.
The adrenal medulla produces compounds with a structure that is far from steroids. They contain a 3,4-dihydroxyphenyl (catechol) nucleus and are called catecholamines. These include adrenaline, noradrenaline, and dopamine beta-hydroxytyramine.
The sequence of catecholamine synthesis is quite simple: tyrosine → dihydroxyphenylalanine (DOPA) → dopamine → norepinephrine → adrenaline. Tyrosine enters the body with food, but can also be formed from phenylalanine in the liver under the action of phenylalanine hydroxylase. The final products of tyrosine conversion in tissues are different. In the adrenal medulla, the process proceeds to the stage of adrenaline formation, in the endings of the sympathetic nerves - norepinephrine, in some neurons of the central nervous system, the synthesis of catecholamines ends with the formation of dopamine.
The conversion of tyrosine to DOPA is catalyzed by tyrosine hydroxylase, whose cofactors are tetrahydrobiopterin and oxygen. It is believed that this enzyme limits the rate of the entire process of catecholamine biosynthesis and is inhibited by the end products of the process. Tyrosine hydroxylase is the main target of regulatory effects on catecholamine biosynthesis.
The conversion of DOPA to dopamine is catalyzed by the enzyme DOPA decarboxylase (cofactor - pyridoxal phosphate), which is relatively non-specific and decarboxylates other aromatic L-amino acids. However, there are indications of the possibility of modifying the synthesis of catecholamines by changing the activity of this enzyme. Some neurons lack enzymes for the further conversion of dopamine, and it is the final product. Other tissues contain dopamine beta-hydroxylase (cofactors - copper, ascorbic acid and oxygen), which converts dopamine to norepinephrine. In the adrenal medulla (but not in the endings of the sympathetic nerves), phenylethanolamine is present - a methyltransferase that forms adrenaline from norepinephrine. In this case, S-adenosylmethionine serves as a donor of methyl groups.
It is important to remember that the synthesis of phenylethanolamine-N-methyltransferase is induced by glucocorticoids entering the medulla from the cortex via the portal venous system. This may explain the fact that two different endocrine glands are combined in one organ. The importance of glucocorticoids for the synthesis of adrenaline is emphasized by the fact that the cells of the adrenal medulla that produce noradrenaline are located around the arterial vessels, whereas the adrenaline-producing cells receive blood mainly from the venous sinuses located in the adrenal cortex.
The breakdown of catecholamines occurs mainly under the influence of two enzyme systems: catechol-O-methyltransferase (COMT) and monoamine oxidase (MAO). The main pathways of adrenaline and noradrenaline breakdown are schematically shown in Fig. 54. Under the influence of COMT in the presence of the methyl group donor S-adrenosylmethionine, catecholamines are converted into normetanephrine and metanephrine (3-O-methyl derivatives of noradrenaline and adrenaline), which, under the influence of MAO, are converted into aldehydes and then (in the presence of aldehyde oxidase) into vanillylmandelic acid (VMA), the main breakdown product of noradrenaline and adrenaline. In the same case, when catecholamines are initially exposed to MAO rather than COMT, they are converted into 3,4-dioxomandelic aldehyde, and then, under the influence of aldehyde oxidase and COMT, into 3,4-dioxomandelic acid and VMC. In the presence of alcohol dehydrogenase, 3-methoxy-4-oxyphenylglycol, which is the main end product of adrenaline and noradrenaline degradation in the CNS, can be formed from catecholamines.
The breakdown of dopamine is similar, except that its metabolites lack the hydroxyl group at the beta-carbon atom, and therefore, instead of vanillylmandelic acid, homovanillic acid (HVA) or 3-methoxy-4-hydroxyphenylacetic acid is formed.
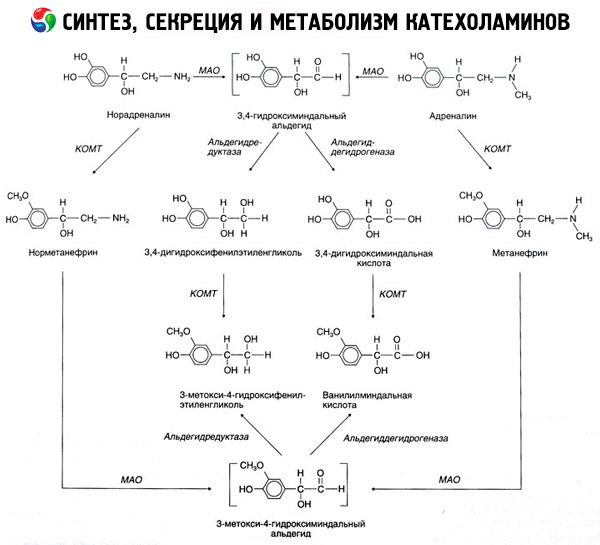
The existence of a quinoid pathway of oxidation of the catecholamine molecule, which may produce intermediate products with pronounced biological activity, is also postulated.
Norepinephrine and adrenaline formed under the action of cytosolic enzymes in the sympathetic nerve endings and the adrenal medulla enter the secretory granules, which protects them from the action of degradation enzymes. The capture of catecholamines by granules requires energy expenditure. In the chromaffin granules of the adrenal medulla, catecholamines are tightly bound to ATP (in a ratio of 4:1) and specific proteins - chromogranins, which prevents the diffusion of hormones from the granules into the cytoplasm.
The direct stimulus for the secretion of catecholamines is apparently the penetration of calcium into the cell, stimulating exocytosis (fusion of the granule membrane with the cell surface and their rupture with the complete release of soluble contents - catecholamines, dopamine beta-hydroxylase, ATP and chromogranins - into the extracellular fluid).
Physiological effects of catecholamines and their mechanism of action
The effects of catecholamines begin with interaction with specific receptors of target cells. While receptors for thyroid and steroid hormones are localized inside cells, receptors for catecholamines (as well as acetylcholine and peptide hormones) are present on the outer cell surface.
It has long been established that in relation to some reactions, adrenaline or noradrenaline are more effective than the synthetic catecholamine isoproterenol, while in relation to others, the effect of isoproterenol is superior to the actions of adrenaline or noradrenaline. On this basis, a concept was developed about the presence of two types of adrenoreceptors in tissues: alpha and beta, and in some of them only one of these two types can be present. Isoproterenol is the most powerful agonist of beta-adrenoreceptors, while the synthetic compound phenylephrine is the most powerful agonist of alpha-adrenoreceptors. Natural catecholamines - adrenaline and noradrenaline - are able to interact with receptors of both types, but adrenaline exhibits a greater affinity for beta-, and noradrenaline - for alpha-receptors.
Catecholamines activate cardiac beta-adrenergic receptors more strongly than smooth muscle beta-receptors, which allowed the beta type to be divided into subtypes: beta1-receptors (heart, fat cells) and beta2-receptors (bronchi, blood vessels, etc.). The effect of isoproterenol on beta1-receptors exceeds the effect of adrenaline and noradrenaline only 10 times, while on beta2-receptors it acts 100-1000 times stronger than natural catecholamines.
The use of specific antagonists (phentolamine and phenoxybenzamine for alpha- and propranolol for beta-receptors) confirmed the adequacy of the classification of adrenoreceptors. Dopamine is capable of interacting with both alpha- and beta-receptors, but various tissues (brain, pituitary gland, vessels) also have their own dopaminergic receptors, the specific blocker of which is haloperidol. The number of beta-receptors varies from 1000 to 2000 per cell. The biological effects of catecholamines mediated by beta-receptors are usually associated with the activation of adenylate cyclase and an increase in the intracellular content of cAMP. Although the receptor and the enzyme are functionally connected, they are different macromolecules. Guanosine triphosphate (GTP) and other purine nucleotides participate in the modulation of adenylate cyclase activity under the influence of the hormone-receptor complex. By increasing enzyme activity, they appear to reduce the affinity of beta receptors for agonists.
The phenomenon of increased sensitivity of denervated structures has long been known. On the contrary, prolonged exposure to agonists reduces the sensitivity of target tissues. The study of beta receptors has made it possible to explain these phenomena. It has been shown that prolonged exposure to isoproterenol leads to a loss of sensitivity of adenylate cyclase due to a decrease in the number of beta receptors.
The process of desensitization does not require activation of protein synthesis and is probably due to the gradual formation of irreversible hormone-receptor complexes. On the contrary, the introduction of 6-oxidopamine, which destroys sympathetic endings, is accompanied by an increase in the number of reacting beta-receptors in tissues. It is possible that an increase in sympathetic nervous activity also causes age-related desensitization of blood vessels and adipose tissue in relation to catecholamines.
The number of adrenoreceptors in different organs can be controlled by other hormones. Thus, estradiol increases and progesterone decreases the number of alpha-adrenoreceptors in the uterus, which is accompanied by a corresponding increase and decrease in its contractile response to catecholamines. If the intracellular "second messenger" formed by the action of beta-receptor agonists is certainly cAMP, then the situation with respect to the transmitter of alpha-adrenergic effects is more complicated. The existence of various mechanisms is assumed: a decrease in the level of cAMP, an increase in the content of cAMP, modulation of cellular calcium dynamics, etc.
To reproduce various effects in the body, doses of adrenaline are usually required that are 5-10 times smaller than noradrenaline. Although the latter is more effective in relation to a- and beta1-adrenoreceptors, it is important to remember that both endogenous catecholamines are capable of interacting with both alpha- and beta-receptors. Therefore, the biological response of a given organ to adrenergic activation largely depends on the type of receptors present in it. However, this does not mean that selective activation of the nervous or humoral link of the sympathetic-adrenal system is impossible. In most cases, increased activity of its various links is observed. Thus, it is generally accepted that hypoglycemia reflexively activates the adrenal medulla, while a decrease in blood pressure (postural hypotension) is accompanied mainly by the release of noradrenaline from the endings of the sympathetic nerves.
Adrenoreceptors and the effects of their activation in various tissues
System, organ |
Adrenergic receptor type |
Reaction |
Cardiovascular system: |
||
Heart |
Beta |
Increased heart rate, conductivity and contractility |
Arterioles: |
||
Skin and mucous membranes |
Alpha |
Reduction |
Skeletal muscles |
Beta |
Expansion Contraction |
Abdominal organs |
Alpha (more) |
Reduction |
Beta |
Extension |
|
Veins |
Alpha |
Reduction |
Respiratory system: |
||
Muscles of the bronchi |
Beta |
Extension |
Digestive system: |
||
Stomach |
Beta |
Decreased motor skills |
Intestines |
Alpha |
Contraction of the sphincters |
Spleen |
Alpha |
Reduction |
Beta |
Relaxation |
|
Exocrine pancreas |
Alpha |
Decreased secretion |
Urogenital system: |
Alpha |
Sphincter contraction |
Bladder |
Beta |
Relaxation of the ejector muscle |
Male genitalia |
Alpha |
Ejaculation |
Eyes |
Alpha |
Pupil dilation |
Leather |
Alpha |
Increased sweating |
Salivary glands |
Alpha |
Excretion of potassium and water |
Beta |
Amylase secretion |
|
Endocrine glands: |
||
Islets of the pancreas |
||
Beta cells |
Alpha (more) |
Decreased insulin secretion |
Beta |
Increased insulin secretion |
|
Alpha cells |
Beta |
Increased secretion of glucagon |
8-cells |
Beta |
Increased secretion of somatostatin |
Hypothalamus and pituitary gland: |
||
Somatotrophs |
Alpha |
Increased secretion of STH |
Beta |
Decreased secretion of STH |
|
Lactotrophs |
Alpha |
Decreased secretion of prolactin |
Thyrotrophs |
Alpha |
Decreased TSH secretion |
Corticotrophs |
Alpha |
Increased ACTH secretion |
| beta | Decreased ACTH secretion | |
Thyroid gland: |
||
Follicular cells |
Alpha |
Decreased secretion of thyroxine |
Beta |
Increased secretion of thyroxine |
|
Parafollicular (K) cells |
Beta |
Increased secretion of calcitonin |
Parathyroid glands |
Beta |
Increased secretion of PTH |
Kidneys |
Beta |
Increased renin secretion |
Stomach |
Beta |
Increased secretion of gastrin |
BX |
Beta |
Increased oxygen consumption |
Liver |
? |
Increased glycogenolysis and gluconeogenesis with the release of glucose; increased ketogenesis with the release of ketone bodies |
Adipose tissue |
Beta |
Increased lipolysis with the release of free fatty acids and glycerol |
Skeletal muscles |
Beta |
Increased glycolysis with the release of pyruvate and lactate; decreased proteolysis with a decrease in the release of alanine, glutamine |
It is important to take into account that the results of intravenous administration of catecholamines do not always adequately reflect the effects of endogenous compounds. This applies mainly to norepinephrine, since in the body it is released mainly not into the blood, but directly into the synaptic clefts. Therefore, endogenous norepinephrine activates, for example, not only vascular alpha receptors (increased blood pressure), but also beta receptors of the heart (increased heart rate), whereas the introduction of norepinephrine from the outside leads mainly to the activation of vascular alpha receptors and a reflex (via the vagus) slowing of the heartbeat.
Low doses of adrenaline activate mainly beta receptors of muscular vessels and the heart, resulting in a decrease in peripheral vascular resistance and an increase in cardiac output. In some cases, the first effect may prevail, and hypotension develops after the administration of adrenaline. In higher doses, adrenaline also activates alpha receptors, which is accompanied by an increase in peripheral vascular resistance and, against the background of an increase in cardiac output, leads to an increase in arterial pressure. However, its effect on vascular beta receptors is also preserved. As a result, the increase in systolic pressure exceeds the similar indicator of diastolic pressure (increase in pulse pressure). With the introduction of even higher doses, the alpha-mimetic effects of adrenaline begin to prevail: systolic and diastolic pressure increase in parallel, as under the influence of norepinephrine.
The effect of catecholamines on metabolism consists of their direct and indirect effects. The former are realized mainly through beta receptors. More complex processes are associated with the liver. Although increased liver glycogenolysis is traditionally considered to be the result of beta receptor activation, there is also evidence of alpha receptor involvement. Indirect effects of catecholamines are associated with modulation of secretion of many other hormones, such as insulin. In the effect of adrenaline on its secretion, the alpha adrenergic component clearly predominates, since it has been shown that any stress is accompanied by inhibition of insulin secretion.
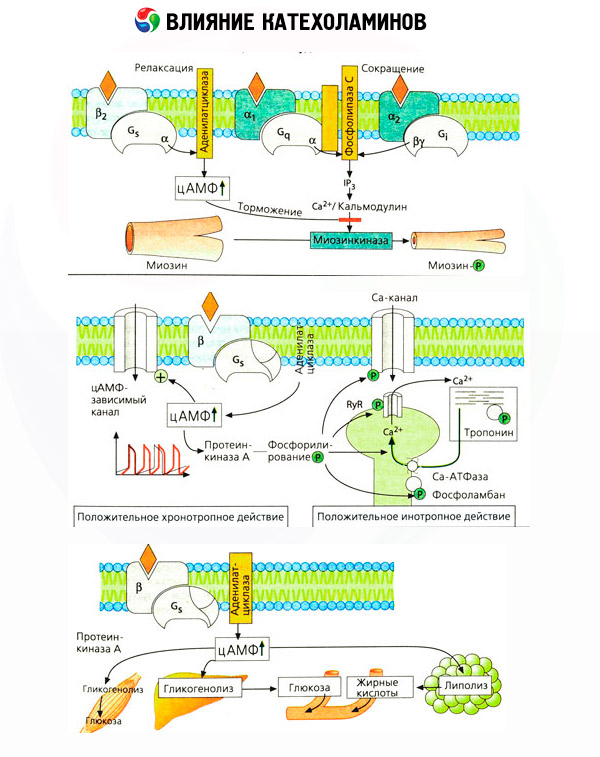
The combination of direct and indirect effects of catecholamines causes hyperglycemia, associated not only with increased hepatic glucose production, but also with inhibition of its utilization by peripheral tissues. Acceleration of lipolysis causes hyperlipacidemia with increased delivery of fatty acids to the liver and intensification of ketone body production. Increased glycolysis in muscles leads to an increase in the release of lactate and pyruvate into the blood, which, together with glycerol released from adipose tissue, serve as precursors of hepatic gluconeogenesis.
Regulation of catecholamine secretion. The similarity of the products and methods of reaction of the sympathetic nervous system and the adrenal medulla was the basis for combining these structures into a single sympathetic-adrenal system of the body with the allocation of its nervous and hormonal links. Various afferent signals are concentrated in the hypothalamus and the centers of the spinal cord and medulla oblongata, from where efferent messages originate, switching to the cellular bodies of preganglionic neurons located in the lateral horns of the spinal cord at the level of the VIII cervical - II-III lumbar segments.
The preganglionic axons of these cells leave the spinal cord and form synaptic connections with neurons located in the ganglia of the sympathetic chain or with cells of the adrenal medulla. These preganglionic fibers are cholinergic. The first fundamental difference between sympathetic postganglionic neurons and chromaffin cells of the adrenal medulla is that the latter transmit the cholinergic signal received by them not by nerve conduction (postganglionic adrenergic nerves), but by the humoral pathway, releasing adrenergic compounds into the blood. The second difference is that postganglionic nerves produce norepinephrine, while cells of the adrenal medulla produce mainly adrenaline. These two substances have different effects on tissues.

