Medical expert of the article
New publications
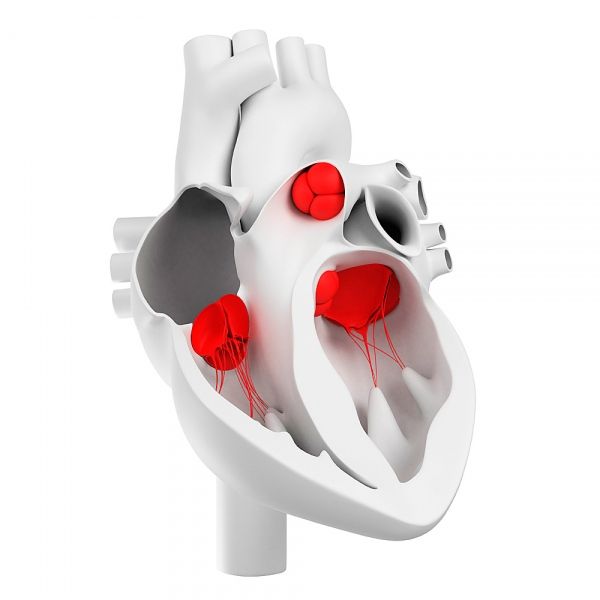
Heart valves
Last reviewed: 04.07.2025

All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.
It was previously believed that all heart valves were simple structures whose contribution to unidirectional blood flow was simply passive movement in response to an applied pressure gradient. This understanding of "passive structures" led to the development of "passive" mechanical and biological valve substitutes.
It is now becoming obvious that heart valves have a more complex structure and function. Therefore, the creation of an "active" heart valve substitute assumes a significant similarity in structure and function with the natural heart valve, which in the future is quite realistic thanks to the development of tissue engineering.
Heart valves develop from embryonic rudiments of mesenchymal tissue during the formation of the endocardium. During morphogenesis, the atrioventricular canal (tricuspid and mitral heart valves) and the ventricular outflow tract (aortic and pulmonary heart valves) are formed.
How are heart valves arranged?
The study of the blood supply to the valves was initiated by N. Luschka (1852), who injected the heart vessels with a contrast mass. He discovered numerous blood vessels in the cusps of the atrioventricular and semilunar valves of the aorta and pulmonary artery. At the same time, a number of manuals on pathological anatomy and histology contained indications that unchanged human heart valves do not contain blood vessels, and the latter appear in the valves only in various pathological processes - atherosclerosis and endocarditis of various etiologies. Information about the absence of blood vessels was based mainly on histological studies. It was assumed that in the absence of blood vessels in the free part of the cusps, their nutrition occurs by filtering fluid from the blood plasma washing the cusps. Penetration of a few vessels together with fibers of striated muscle tissue into the bases of the valves and tendinous chords was noted.
However, when injecting cardiac vessels with various dyes (Indian ink in gelatin, bismuth in gelatin, aqueous suspension of black Indian ink, solutions of carmine or trypan blue), it was found that the vessels penetrate the atrioventricular heart valves, the aortic valves and the pulmonary artery along with the cardiac muscle tissue, slightly short of reaching the free edge of the valve.
In the loose fibrous connective tissue of the atrioventricular valve cusps, individual main vessels were found that anastomosed with vessels in adjacent areas of cardiac striated muscle tissue.
The largest number of blood vessels were located at the base and a comparatively smaller number in the free part of these valves.
According to K. I. Kulchitsky et al. (1990), a larger diameter of arterial and venous vessels is found in the mitral valve. At the base of the cusps of this valve are located mainly the main vessels with a narrow-loop network of capillaries, penetrating into the basal part of the cusp and occupying 10% of its area. In the tricuspid valve, the arterial vessels have a smaller diameter than in the mitral valve. In the cusps of this valve, there are mainly scattered vessels and comparatively wide loops of blood capillaries. In the mitral valve, the anterior cusp is supplied with blood more intensively, in the tricuspid valve - the anterior and posterior cusps, which perform the main closing function. The ratio of the diameters of the arterial and venous vessels in the atrioventricular valves of the heart of mature people is 1:1.5. The capillary loops are polygonal and are located perpendicular to the base of the valve cusps. The vessels form a planar network located under the endothelium on the atrial side. Blood vessels are also found in the tendinous chords, where they penetrate from the papillary muscles of the right and left ventricles at a distance of up to 30% of the length of the tendinous chords. Numerous blood vessels form arcuate loops at the base of the tendinous chords. The heart valves of the aorta and pulmonary trunk differ significantly from the atrioventricular valves in terms of blood supply. The main vessels of a relatively smaller diameter approach the base of the semilunar cusps of the aorta and pulmonary trunk valves. The short branches of these vessels end in capillary loops of irregular oval and polygonal shape. They are located mainly near the base of the semilunar cusps. The venous vessels at the base of the aortic and pulmonary valves also have a smaller diameter than those at the base of the atrioventricular valves. The ratio of the diameters of the arterial and venous vessels in the aortic and pulmonary valves of the heart of mature people is 1:1.4. Short lateral branches extend from the larger vessels, ending in loops of capillaries of irregular oval and polygonal shapes.
With age, there is a coarsening of connective tissue fibers, both collagen and elastic, as well as a decrease in the amount of loose fibrous unformed connective tissue, sclerosis of the tissue of the atrioventricular valve cusps and the semilunar cusps of the aortic and pulmonary artery valves develops. The length of the cardiac striated muscle fibers in the valves decreases, and, consequently, its quantity and the number of blood vessels penetrating the heart valves decrease. Due to these changes, the heart valves lose their elastic and resilient properties, which affects the mechanism of valve closure and hemodynamics.
The heart valves have networks of lymphatic capillaries and a small number of lymphatic vessels equipped with valves. The lymphatic capillaries of the cusps have a characteristic appearance: their lumen is very uneven, the same capillary in different areas has a different diameter. In places where several capillaries merge, expansions are formed - lacunae of various shapes. The loops of the networks are often irregular polygonal, less often oval or round. Often the loops of the lymphatic networks are not closed, and the lymphatic capillaries end blindly. The loops of the lymphatic capillaries are oriented most often in the direction from the free edge of the cusp to its base. In some cases, a two-layer network of lymphatic capillaries was found in the cusps of the atrioventricular valve.
The endocardial nerve plexuses are located in its various layers, mainly under the endothelium. At the free edge of the valve cusps, the nerve fibers are located mainly radially, connecting with those of the tendinous chordae. Closer to the base of the cusps, a large-meshed nerve plexus is formed, which connects with the plexus located around the fibrous rings. On the semilunar cusps, the endocardial nerve network is more sparse. At the place of attachment of the valves, it becomes dense and multilayered.
Cellular structure of heart valves
Valvular interstitial cells, responsible for maintaining the structure of the valve, are elongated in shape with numerous fine processes that extend throughout the valve matrix. There are two populations of valvular interstitial cells that differ in morphology and structure; one has contractile properties and is characterized by the presence of contractile fibrils, the other has secretory properties and has a well-developed endoplasmic reticulum and Golgi apparatus. Contractile function resists hemodynamic pressure and is further supported by the production of both cardiac and skeletal contractile proteins, which include the heavy chains of alpha- and beta-myosin and various isoforms of troponin. Contraction of the cardiac valve leaflet has been demonstrated in response to a number of vasoactive agents, suggesting a coordinated biological stimulus for successful valve function.
Interstitial cells are also essential components of the repair system of structures such as heart valves. The constant movement of the valve leaflets and the associated connective tissue deformation produces damage to which valvular interstitial cells respond in order to maintain the integrity of the valve. The repair process appears to be vital for normal valve function, and the absence of these cells in current artificial valve models is likely a contributing factor to the structural damage of bioprostheses.
An important area of research in interstitial cells is the study of interactions between them and the surrounding matrix mediated by focal adhesion molecules. Focal adhesions are specialized cell-matrix interaction sites that link the cell cytoskeleton to matrix proteins via integrins. They also act as signal transduction sites, relaying mechanical information from the extracellular matrix that can elicit responses including, but not limited to, cell adhesion, migration, growth, and differentiation. Understanding the cell biology of valvular interstitial cells is vital to elucidating the mechanisms by which these cells interact with each other and their environment, so that this function can be recapitulated in artificial valves.
In connection with the development of a promising direction of tissue engineering of heart valves, studies of interstitial cells are carried out using a wide range of techniques. The presence of the cell cytoskeleton is confirmed by staining for vimentin, desmin, troponin, alpha-actin and smooth muscle myosin, heavy chains of alpha- and beta-myosin, light chains-2 of cardiac myosin, alpha- and beta-tubulin. Cell contractility is confirmed by a positive response to epinephrine, angiotensin II, bradykinin, carbachol, potassium chloride, endothelium I. Cellular interrelationships are determined by functional gap interactions and verified by microinjections of carboxyfluorescein. Matrix secretion is established by staining for prolyl-4-hydroxylase / collagen type II, fibronectin, chondroitin sulfate, laminin. Innervation is established by the close location of motor nerve endings, which is reflected by the activity of neuropeptide Y tyrosine hydroxylase, acetylcholinesterase, vasoactive intestinal polypeptide, substance-P, capsicum gene-related peptide. Mitogenic factors are estimated by platelet-derived growth factor, basic fibroblast growth factor, serotonin (5-HT). The studied interstitial cell fibroblasts are characterized by an incomplete basement membrane, long, thin cytoplasmic processes, close connection with the matrix, well-developed uneven endoplasmic reticulum and Golgi apparatus, richness in microfilaments, formation of adhesive bonds.
Valvular endocardial cells form a functional athrombogenic sheath around each heart valve similar to the vascular endothelium. The widely used method of valve replacement eliminates the protective function of the endocardium, which may lead to platelet and fibrin deposition on artificial valves, development of bacterial infection and tissue calcification. Another probable function of these cells is the regulation of the underlying valvular interstitial cells similar to the regulation of smooth muscle cells by the endothelium. Complex interactions exist between the endothelium and adjacent cells, mediated in part by soluble factors secreted by the endothelial cells. These cells form a huge surface covered with microprotrusions on the luminal side, thus increasing the exposure and possible interaction with metabolic substances in the circulating blood.
The endothelium often displays morphological and functional differences caused by shear stresses on the vessel wall due to blood flow, and this also applies to the valvular endocardial cells, which adopt either an elongated or polygonal shape. Changes in cell structure may occur due to the action of local hemodynamics on components of the cell cytoskeleton or secondary effects caused by changes in the underlying extracellular matrix. At the ultrastructural level, valvular endocardial cells possess intercellular connections, plasma vesicles, a rough endoplasmic reticulum, and a Golgi apparatus. Although they produce von Willebrand factor both in vivo and in vitro, they lack Weibel-Palade bodies (specific granules containing von Willebrand factor), which are organelles characteristic of the vascular endothelium. Valvular endocardial cells are characterized by strong junctions, functional gap interactions, and overlapping marginal folds.
Endocardial cells retain their metabolic activity even in vitro: they produce von Willebrand factor, prostacyclin, nitric oxide synthase, demonstrate angiotensin-converting enzyme activity, and intensively secrete adhesion molecules ICAM-1 and ELAM-1, which are important for binding mononuclear cells during the development of an immune response. All these markers should be taken into account when growing an ideal cell culture for creating an artificial valve using tissue engineering, but the immunostimulating potential of valvular endocardial cells themselves may limit their use.
The extracellular matrix of the heart valves consists of fibrous collagen and elastin macromolecules, proteoglycans and glycoproteins. Collagen accounts for 60% of the dry weight of the valve, elastin for 10% and proteoglycans for 20%. The collagen component provides the main mechanical stability of the valve and is represented by collagens of types I (74%), II (24%) and V (2%). Bundles of collagen threads are surrounded by an elastin sheath, which mediates interactions between them. The glycosaminoglycan side chains of proteoglycan molecules tend to form a gel-like substance in which other matrix molecules interact to form permanent bonds and other components are deposited. Glycosaminoglycans of human heart valves consist mainly of hyaluronic acid, to a lesser extent of dermatan sulfate, chondroitin-4-sulfate and chondroitin-6-sulfate, with a minimal amount of heparan sulfate. Remodeling and renewal of matrix tissue are regulated by matrix metalloproteinases (MMPs) and their tissue inhibitors (TIs). These molecules are also involved in a wider range of physiological and pathological processes. Some metalloproteinases, including interstitial collagenases (MMP-1, MMP-13) and gelatinases (MMP-2, MMP-9) and their tissue inhibitors (TI-1, TI-2, TI-3), are found in all heart valves. Excess production of metalloproteinases is characteristic of pathological conditions of the heart valve.
 [ 6 ], [ 7 ], [ 8 ], [ 9 ], [ 10 ], [ 11 ], [ 12 ], [ 13 ], [ 14 ], [ 15 ], [ 16 ]
[ 6 ], [ 7 ], [ 8 ], [ 9 ], [ 10 ], [ 11 ], [ 12 ], [ 13 ], [ 14 ], [ 15 ], [ 16 ]
Heart valves and their morphological structure
Heart valves consist of three morphologically different and functionally significant layers of the leaflet matrix: fibrous, spongy, and ventricular.
The fibrous layer forms a load-resistant framework for the valve leaflet, consisting of layers of collagen fibers. These fibers are arranged radially in folds to allow the arterial valves to stretch when closing. The fibrous layer lies near the outlet outer surface of these valves. The fibrous layer of the atrioventricular valves serves as a continuation of the collagen bundles of the chordae tendineae. It is located between the spongy (inlet) and ventricular (outlet) layers.
Between the fibrous and ventricular layers is the spongy layer (spongiosa). The spongy layer consists of poorly organized connective tissue in a viscous medium. The dominant matrix components of this layer are proteoglycans with randomly oriented collagen and thin layers of elastin. The side chains of proteoglycan molecules carry a strong negative charge, which affects their high ability to bind water and form a porous matrix gel. The spongy layer of the matrix reduces mechanical stress in the heart valve leaflets and maintains their flexibility.
The ventricular layer is much thinner than the others and is rich in elastic fibers that allow the tissue to resist constant deformation. Elastin has a spongy structure surrounding and connecting collagen fibers and maintains them in a neutral folded state. The inlet layer of the valve (ventricular - for arterial valves and spongy - for atrioventricular) contains more elastin than the outlet, which provides for the softening of the hydraulic shock when the cusps close. This relationship between collagen and elastin allows the cusps to stretch up to 40% without stable deformation. When exposed to a small load, the collagen structures of this layer are oriented in the direction of the load, and its resistance to further load growth increases.
Thus, the idea of the heart valves as simple endocardial duplications is not only simplified but also essentially incorrect. Heart valves are complex organs that include striated muscle fibers, blood and lymphatic vessels, and nerve elements. Both in their structure and in their functioning, the valves are integral to all cardiac structures. Analysis of normal valve function must take into account its cellular organization, as well as the interaction of cells with each other and the matrix. The knowledge gained from such studies is leading in the design and development of valve prosthetics using tissue engineering.

