Medical expert of the article
New publications
Glioma of the brain
Last reviewed: 29.06.2025

All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.
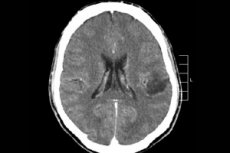
Among the many tumor processes of the central nervous system, brain glioma is most often diagnosed - this term is collective, the neoplasm combines all diffuse oligodendroglial and astrocytic foci, astrocytoma, astroblastoma and so on. Such a tumor can have a different degree of malignancy, is formed from glial structures - cells localized around neurons. The main area of location of gliomas are cerebral hemispheres, walls of the brain ventricles and chiasma - the area of partial intersection of optic nerve fibers. Externally, the tumor is a nodular element of pinkish or reddish hue, round or spindle-shaped configuration with indistinct boundaries. [1]
Epidemiology
In about 5% of cases, gliomas are associated with hereditary pathologies - in particular, neurofibromatosis and other syndromes with dominant inheritance. Experts point out that the absolute majority of brain gliomas develop sporadically - that is, without a clear cause.
Overall, primary neoplasms of the central nervous system account for approximately 2% of all tumors, or just over 21 cases per hundred thousand population. Among them, gliomas occur in 35-36% of cases, and more than 15% of them are glioblastomas.
According to some data, glioma affects men more often than women - the tumor is especially common among people over 50 years old.
The global incidence of gliomas among the elderly has increased significantly in recent decades. The reasons for this phenomenon have not yet been established.
According to the definition of the World Health Organization, three main variants of glial tumors, differing in their histological characteristics, have been identified. These are oligodendrogliomas, astrocytomas and combined oligoastrocytomas. The incidence of each subtype of low malignant pathology has not been reliably determined. Some studies indicate an increase in the incidence of oligodendrogliomas from 5% to 30% and a decrease in the incidence of astrocytomas.
Glial tumors are capable of infiltrating brain tissue, and the vast majority of low-grade foci become malignant within a few years. [2]
Causes of the brain gliomas
Brain glioma is a whole group of tumor processes, the common feature of which is their formation from glial structures of the CNS located in the brain tissue. Such tumors are divided into two histopathological variants: high malignant and low malignant gliomas.
The source of growth formation is neuroglia cells (astrocytes, oligodendrocytes), which provide the structural basis and viability of brain neurons.
Glial tumor processes differ greatly in structure, mutational changes in genes, aggressiveness, clinical features, diagnostic characteristics, response to treatment, and prognosis of patients. Embryonal and ependymal neoplasms of the central nervous system - in particular, medulloblastomas and ependymomas - differ in their histologic structure but are similar in terms of treatment.
Glial elements were first classified as a separate structural category of the nervous system in the late 19th century.
Neuroglia tissue consists of cells that have auxiliary functions: trophic, support, protective, secretory. Neurons and gliocytes exist together with each other, they together form the nervous system and are of great importance in the general processes of the organism's vital activity.
Gliocytes are roughly categorized into several major forms: astrocytes, oligodendrocytes, ependymal cells, and microglia.
To date, scientists cannot answer the question about the reliable causes of neuroglial tumor formation. Presumably, a certain negative contribution is made by radioactive effects, infectious diseases, intoxication (especially chemical, occupational). The hereditary factor is also important.
Brain gliomas arise from abnormal neurogliocytes that have a genetic defect that leads to abnormal growth and functionality - such structures are referred to as "immature". Incomplete cells are more often located in one area, where the tumor is formed.
Simply put, glial formation is the result of chaotic and sporadic growth of modified neuroglia cells. The process can develop from ependymocytes, oligodendrocytes, astrocytes (astrocytoma, including giant cell and anaplastic). [3]
Risk factors
Despite the fact that experts cannot accurately characterize the causes of the formation of glial oncopathologies, in some cases their appearance can be prevented by eliminating the main risk factors:
- Ionizing radiation exposure has carcinogenic activity, can cause the development of leukemia and the formation of cancer processes with a dense structure, including in persons of young age. Frequent and unreasonable radiological medical procedures, ultraviolet radiation (including solarium) also belong to potential carcinogenic effects and can cause the appearance of tumors in various organs, including the brain.
- Occupational adverse effects, intoxications often have a causal link with the development of cancerous tumors. The production of rubber and glass, pesticides and fuels, metals and textiles, paints and laboratory reagents are considered particularly dangerous. At risk are workers in the aerospace, coal and metal industries, chemical and by-product manufacturing plants, building materials and electrodes, fuels and lubricants, plastics and monomers.
- Air, water and soil pollution are responsible for up to 4% of all cancer pathologies in the world. Carcinogens, present in large quantities in the environment, enter the body with inhaled air, drinking water and food. Living in ecologically unsafe areas - near large industrial facilities, busy transportation interchanges - is considered especially dangerous.
- Infectious pathologies - in particular viral infections - can also create conditions favorable for the development of tumors. It is important to keep this in mind and to be vaccinated in advance, as well as to prevent infectious and parasitic diseases.
- Tobacco and alcohol intoxication are considered risk factors for many varieties of cancer, not just brain gliomas.
- Insufficient physical activity, overweight, improper nutrition, metabolic disorders, head injuries, vascular pathologies - additional stress factors that can provoke the start of intracellular disorders.
- Older age is the most common period for the development of neoplasms in the body, so those over 55 years of age should take special care of their own health.
However, the main and most significant risk factor for glioma development remains hereditary predisposition.
Pathogenesis
To date, experts have a number of assumptions regarding the development of brain gliomas. Each theory has its own grounds, but the only correct and reliable pathogenetic mechanism scientists have not yet identified. In most cases, we are talking about the following factors in the development of neoplasms:
Embryogenesis failure, which consists in the disruption of organ laying and the formation of "wrong" cell structures;
- Exposure to ionizing rays, potential carcinogens in the form of chemical agents, food additives, etc.;
- Head trauma;
- Gene disorders passed on from generation to generation ("familial" glioma);
- Immune dysfunction, neuroinfections.
Most gliomas have diffuse growth, with penetration into the surrounding normal brain tissue. Depending on the degree of malignancy, the tumor can develop for several years without any manifestation. In case of aggressive course, the symptomatology rapidly increases over several months.
Part of tumorigenesis is due to dysembryogenetic changes.
The brainstem can be affected at different levels: diffuse brainstem glioma, in turn, will differ both anatomo-morphologically and clinically. Some such neoplasms - in particular, glioma of the quadriplegia plate - can be relatively benign, with no signs of progression. A pontine glioma, on the other hand, is characterized by its particular malignancy, aggressiveness, and poor prognosis.
Diffuse lesions of brain structures, in which more than three anatomical zones of the large hemispheres are involved in the pathologic process, with possible periventricular divergence and passage through the corpus call gliomatosis. [4]
Is brain glioma hereditary?
A well-proven risk of brain glioma formation is hereditary - that is, the presence of similar or other intracerebral tumors in direct ancestors or in the same generation. Radioactive exposures and regular or prolonged contact with potential carcinogens exacerbate the situation.
Not only can gliomas be inherited, but also diseases that are accompanied by increased tumor growth without reference to the localization - in particular, this may be neurofibromatosis type 1 and 2, Li-Fraumeni syndrome, Hippel-Lindau. Often in glioma cells, changes in certain genes or chromosomes are detected.
The main pathologies that are associated with the development of glioma in humans are summarized in the table:
|
Pathology |
Chromosome |
Gene |
Variety of neoplasm |
|
Li-Fraumeni syndrome |
17р13 |
TR53 |
Neuroectodermal neoplasms, astrocytoma. |
|
Neurofibromatosis |
17q11 |
NF1 |
Optic nerve glioma, pilocytic astrocytoma, neurofibromatosis |
|
Turcotte's syndrome |
3p21, 7p22 |
HMLH1, HPSM2 |
Astrocytoma |
|
Tuberous sclerosis (Burneville's syndrome). |
9q34, 16p13 |
TSC1, TSC2 |
Gigantocellular subependymal astrocytoma |
Regardless of the nature of the glial tumor, whether it is a sporadic case or a hereditary pathology, it is a disorder with the expression of a pathologically altered gene. Apart from neoplasms formed as a result of learning effects, in other situations the causes of genetic alterations remain unclear.
Symptoms of the brain gliomas
Features of focal symptomatology directly depend on the area of localization of brain glioma and become a consequence of all sorts of endocrine disorders, compression of nerve tissue or local destructive processes.
If the neoplasm is located in the parietal zone, then a person is dominated by such manifestations as seizures, sensory disorders, hearing impairment.
When the glioma is localized in the area of the dominant hemisphere, speech disorders, agraphia, agnosia are detected.
Temporal lobe neoplasms are often accompanied by convulsive seizures, aphasia, impaired sense of smell and visual function, and dyspnea.
When intracranial pressure increases, a corresponding picture develops with restriction of visual fields, paralysis of eye muscles, and hemiplegia.
Due to the specificity of the tumor process, brain glioma is always accompanied by neurological symptoms to a greater or lesser extent. At first, there is a noticeable general weakness, the patient constantly wants to sleep, ability to work is impaired, thought processes are slowed down. It is at this stage that there is a high risk of making an incorrect diagnosis and, as a consequence, prescribing the wrong treatment. Among other nonspecific manifestations:
- Vestibular disorders, including unsteady gait, loss of balance (e.g., when bicycling or climbing stairs), numbness in the limbs, etc;
- Gradual deterioration of vision, doubling of the visual picture;
- Deterioration of auditory function;
- Slurred speech;
- Nausea and vomiting in the form of attacks independent of food or drink;
- Weakening of the mimic muscles and other facial muscles;
- Discomfort when swallowing;
- Regular headaches (often in the morning hours).
The clinical picture gradually expands and worsens: in some patients it happens slowly, in others - abruptly, literally "before their eyes", within a few weeks. In the latter case, we are talking about an aggressive, rapidly developing glioma of the brain.
First signs
Glioma of the brain in the early stages of development does not have a pronounced symptomatology. The first manifestations are often mistaken for signs of other, less dangerous pathologies.
In general, the clinical picture of glioma is diverse and is determined by the location and size of the pathologic focus. As the neoplasm grows, general cerebral symptoms develop and increase:
- Persistent and regular head pain that does not respond to standard medications (non-steroidal anti-inflammatory drugs);
- Intermittent nausea, sometimes to the point of vomiting;
- An uncomfortable, heavy feeling in the eyeball area;
- Seizures.
Cerebral manifestations are especially intense when the tumor grows into the ventricles or the liquor system. Cerebrospinal fluid drainage is impaired, intracranial pressure increases, and hydrocephalus develops. The process affects a certain part of the brain, which affects the development of the corresponding clinic:
- There are problems with visual function;
- Speech impaired;
- Vestibular disorders (dizziness, impaired coordination of movements) occur;
- Paresis, paralysis of the arms, legs;
- Memory and concentration are impaired;
- Thought processes are impaired;
- Behavioral disorders are emerging.
At the initial stage, the symptoms are practically absent, or they are so insignificant that they do not attract attention. It is for this reason that experts strongly advise regular preventive examinations and checkups. After all, the earlier the tumor process is detected, the greater the chances of cure and survival. [5]
Glioma of the brain in a child
Among the many brain tumors found in childhood, the percentage of gliomas ranges from 15 to 25%. Children can get the disease in their early teens and early twenties, although it is very rare for babies under 3 years of age to get the disease.
The pathology starts against the background of mutation of glial cells. To date, there is no answer to the question of why this mutation occurs.
The only thing that has been reliably learned is that certain inherited diseases associated with an increased risk of tumor growth increase the likelihood of developing brain glioma as well.
In addition, scientists have found that glial cells can have divergence in individual genes or chromosomes. Because of this disorder, a mutation mechanism kicks in, which is not hereditary. It is possible that this occurs at one of the earliest stages of development.
It is a proven fact that the presence of acute leukemia or retinoblastoma in the child's history, or brain irradiation for any other reason, significantly increases the risks of glioma formation (after a certain period of time).
Symptomatology in childhood depends on the degree of malignancy and localization of the pathological focus. A distinction is made between specific and nonspecific symptoms:
- Nonspecific symptoms are not "tied" to the area where the glioma is located. Common manifestations may include head pain, dizziness, poor appetite, vomiting without connection with food intake, weight loss (for unknown reasons), constant feeling of fatigue, drop in academic performance, difficulty in concentration, behavioral disorders. These signs are due to compression of intracranial structures, which can be explained as a direct pressure of the growing mass, and a disorder in the circulation of cerebrospinal fluid. There is a risk of cerebral hydrocele.
- The specific symptomatology depends on the immediate location of the glial pathologic focus. For example, cerebellar tumor is usually accompanied by impaired gait and balance in children. The lesion of the large brain is manifested by convulsive seizures, and tumor growth in the spinal cord - paralysis of the musculature. It happens that the baby's vision deteriorates sharply, consciousness is disturbed, sleep is impaired, or some other developmental problem occurs.
As a rule, in childhood, malignant glioma reveals itself in a few weeks or months of its development: often characterized by rapid and uncontrolled growth of the neoplasm.
Children with malignant glial tumors are treated by doctors at pediatric clinical centers specializing in pediatric oncology. As a rule, surgical treatment, radiation and chemotherapy courses are used.
The most important treatment step is neurosurgery. The more radical it is, the better the child's chances for a cure. But surgical intervention is not always possible: in particular, problems may arise with the removal of brainstem gliomas, as well as with radiation for children under 3 years of age.
Gliomas of the central brain (intermediate and midbrain) are difficult to remove completely, as there is a risk of damage to healthy tissue. If complete resection of the tumor is impossible, the patient is prescribed palliative treatment.
Children with malignant gliomas are treated according to standardized protocols that have been determined through rigorously controlled clinical trials. The most common protocols are as follows:
- HIT HGG 2007: involves the treatment of children aged 3-17 years.
- HIT SKK: suitable for infants (up to three years of age) and does not involve radiation treatments.
Pediatric survival statistics for gliomas are generally not very optimistic. However, in no case is it possible to predict in advance the effectiveness of treatment measures for a particular child. It is important to carefully follow all doctor's orders, which significantly increases the chances of recovery.
Forms
Gliomas can be low malignant and high malignant, with intense growth and a propensity to metastasize. It is important to understand that low malignancy is not synonymous with tumor safety. Any brain neoplasm creates additional volume, squeezes brain structures, which leads to their displacement and increased intracranial pressure. As a result, the patient may die.
There are two main types of malignant astrocytomas. These are glioblastomas and anaplastic astrocytomas, which are subdivided according to molecular changes. Secondary malignant tumors that developed from astrocytomas and have a low degree of malignancy are most often found in young patients. Initially malignant glial-type tumors occur more often in elderly patients.
Depending on the structural location, gliomas come in:
- Supratentorial (with localization above the cerebellum in the area of lateral ventricles, large hemispheres);
- Subtentorial (with localization below the cerebellum in the posterior cranial fossa).
According to histological features, distinguish such types of gliomas:
- Astrocytic glioma is the most common. In turn, it is subdivided into nodular and diffuse (the latter can be characterized by rapid growth and a stroke pattern).
- Oligodendroglioma - occurs in 5% of patients. It has petrificates - areas of calcification, most often in the frontal lobe.
- Ependymal glioma - grows from the structures lining the walls of the central canal of the spinal cord and ventricles. Often grows into the thickness of the brain substance, as well as into the lumen of the brain.
Mixed pathologic foci such as subependymoma, oligoastrocytoma, etc. Are also possible.
All gliomas are categorized into the following stages:
- Slow-growing relatively benign neoplasms without obvious clinical symptoms.
- Slow-growing "borderline" gliomas that gradually transform into stage III and beyond.
- Malignant glioma.
- Malignant glioma with intense aggressive growth and spread, with poor prognosis.
The lower the stage of malignancy, the less probability of metastasis and recurrence of the removed neoplasm, and the greater the chances of cure of the patient. The greatest danger is posed by glioblastoma multiforme, a low-differentiated process with intensive growth and development. [6]
Possible and most common variants of neuroglioma:
- Glioma with brainstem and pontine lesions is located in the area where the brain connects to the spinal cord. It is there that important neurocenters responsible for respiratory, cardiac, and motor function are localized. If this zone is damaged, the work of the vestibular and speech apparatus is disturbed. It is often detected in childhood.
- Visual glioma affects neuroglial cells that surround the optic nerve. The pathology causes visual impairment and exophthalmos. It develops more often in children.
- Low malignant neuroglioma is characterized by slow growth, localized more often in the large hemispheres and cerebellum. It occurs more often in young people (adolescents and young adults around 20 years of age).
- Glioma of the corpus callosum is more characteristic of individuals between 40 and 60 years of age and is most commonly represented by glioblastoma.
- Glioma of the chiasma is localized in the optic junction zone, so it is accompanied by myopia, visual field loss, occlusive hydrocephalus, and neuroendocrine disorders. It can occur at any age, but most commonly affects patients with neurofibromatosis type I.
Complications and consequences
Gliomas of low malignancy (Grade I-II, highly malignant - e.g., astrocytoma, oligoastrocytoma, oligodendroglioma, pleomorphic xanthoastrocytoma, etc.) and high malignancy (Grade III-IV - glioblastoma, anaplastic oligodendroglioma, oligoastrocytoma, and astrocytoma). Grade IV gliomas are particularly malignant.
Brainstem glioma has a very unfavorable prognosis precisely because the neoplasm affects such a brain region, where the most important nerve connections between the brain and the limbs are concentrated. Even a rather small tumor in this area is enough to make the patient's condition quickly deteriorate and provoke paralysis.
No less unfavorable consequences occur when other brain regions are affected. Often it is a tumor of the cerebral cortex, which does not give a chance for a long life expectancy of the patient, despite the treatment. It is often only possible to postpone death.
According to medical statistics, the five-year survival rate is often only 10-20%. Although these figures largely depend on both the degree of malignancy and the exact localization and volume of surgical intervention performed. After complete removal of the pathologic focus, the survival rate increases significantly (sometimes - up to 50%). Lack of treatment or its impossibility (for one reason or another) is guaranteed to lead to the death of the patient.
The majority of low malignant glial tumors are able to infiltrate brain tissue and malignize over several years.
The risk of glioma recurrence is considered by experts to be "highly probable". Nevertheless, treatment should not be neglected: it is important to ensure a good quality of life for as long as possible.
Recurrent gliomas always have a worse prognosis than primary tumors. However, modern treatment protocols based on therapeutic optimization studies often achieve sufficiently good results for patients even with highly malignant neoplasms.
Possible outcomes after chemotherapy:
- Emaciation, emaciation, digestive disorders, oral diseases;
- Increased excitability of the central nervous system, asthenia;
- Deterioration of hearing function, tinnitus and ringing in the ears;
- Seizures, depressive disorders;
- Hypertensive crisis, change in blood pattern;
- Renal failure;
- Allergic processes, hair loss, appearance of pigment spots on the body.
After chemotherapy, patients note a pronounced weakening of the immune system, which can cause the development of various infectious pathologies.
Diagnostics of the brain gliomas
A brain glioma can be suspected by the following signs:
- The patient has localized or generalized seizures, which are characteristic of the cortical location of the neoplasm and its slow development. Epi-seizures are found in 80% of patients with low-grade glial tumors and in 30% of patients with high-grade gliomas.
- Increased intracranial pressure is particularly characteristic of masses located in the right frontal and parietal lobes. Associated with high intracranial pressure disorder of blood circulation and liquor circulation entails the appearance of constant and increasing head pain, nausea with vomiting, visual disturbances, drowsiness. There is edema of the optic nerve, paralysis of the diverting nerve. An increase in intracranial pressure to critical values can lead to the development of coma and death. Another cause of high IOP is hydrocephalus.
- The patient has a growing focal picture. In supratentorial formations, the motor and sensory spheres are disturbed, hemiopia, aphasia, and cognitive disorders progress.
If the doctor suspects the presence of a brain neoplasm, it is optimal to perform MRI without or with the introduction of contrast agent (gadolinium) to find out its location, size, and additional characteristics. If magnetic resonance imaging is not possible, computed tomography is performed, and magnetic resonance spectroscopy is used as a method of differentiation. Despite the informativeness of these diagnostic methods, the final diagnosis is made only after histological confirmation during resection of the tumor focus.
Given the above criteria, it is recommended to begin diagnosis with a thorough history, assessment of somato-neurologic status and functional status. Neurologic status is assessed along with the determination of probable intellectual and mnestic disorders.
Recommended laboratory tests:
- A full-blown general clinical blood workup;
- A full blood chemistry panel;
- Urinalysis;
- Blood coagulation study;
- Analysis for oncologic markers (AFP, beta-hCG, LDH - relevant if a lesion of the pineal zone is suspected).
To clarify prognostic points in patients with glioblastoma and anaplastic astrocytoma, IDH1|2-1 gene mutation and MGMT gene methylation are evaluated. In patients with oligodendroglioma and oligoastrocytoma, 1p|19q codlelation is determined.
Instrumental diagnostics, first of all, is represented by obligatory magnetic resonance imaging of the brain (sometimes - and spinal cord). MRI is performed in three projections using standard T1-2, FLAIR, T1 modes with contrast.
When indicated, ultrasound of the vascular network, functional magnetic resonance imaging of the motor and speech sections, as well as angiography, spectroscopy, MR tractography and perfusion are performed.
Additional investigations may include:
- Electroencephalography of the brain;
- Consultations with a neurosurgeon, oncologist, radiologist, ophthalmologist, radiologist.
Differential diagnosis
Differential diagnosis is necessarily carried out with non-tumor pathologies - in particular, with hemorrhage caused by arterio-venous or arterial malformation, as well as with pseudotumor demyelinating processes, inflammatory diseases (toxoplasmosis, brain abscess, etc.).
In addition, differentiate the primary tumor focus and central nervous system metastases.
With modern magnetic resonance imaging capabilities, it is possible to perform diagnostic measures accurately enough, to find out the origin of the primary focus in the CNS. MRI of the brain is performed with or without contrast, in T1, T2 FLAIR mode - in three projections, or thin slices in axial projection (SPGR mode). These diagnostic methods allow to accurately determine the location, size, structural characteristics of the neoplasm, its relationship with the vascular network and nearby brain areas.
Additionally, CT (with or without contrast), CT angiography (MR angiography), MR tractography, MR or CT perfusion may be performed as part of the differential diagnosis. CT/PET of the brain with methionine, choline, tyrosine, and other amino acids is used when indicated.
Treatment of the brain gliomas
Specific therapy consists of surgical, chemotherapeutic and radiation measures. It is mandatory, if possible, to perform a complete resection of the tumor focus, which allows for rapid symptom relief and histological confirmation of the diagnosis.
Irradiation has a positive effect on increasing the life expectancy of patients. A total dose of 58 to 60 Gy, divided into individual irradiation doses of 1.8-2 Gy, is administered as standard. The tumor is irradiated locally, additionally capturing up to 3 cm around it. Radiation therapy is more acceptable as opposed to brachytherapy. In some cases, radiosurgical methods are recommended, which consist of irradiation with a Gamma Knife or linear gas pedal, as well as neutron-capture boron therapy.
The need for adjuvant chemotherapy is controversial. In some cases, nitrosourea preparations allowed to increase the life expectancy of patients up to one and a half years, but some results of using such chemopreparations were negative. Today, cytotoxic agents, neoadjuvant therapy (before radiation), combined drugs, intra-arterial chemotherapy, or high-dose chemotherapy with further stem cell transplantation are actively used.
In general, for successful treatment of gliomas, a comprehensive approach is very important, the extent of which depends on the location and degree of malignancy of the mass, its size and the general health of the patient.
In relation to brainstem glioma, surgical intervention is rarely used. The main contraindication to surgery is the area of localization of the focus - in close proximity to vital parts. In some cases, it is possible to remove glioma of the trunk using microsurgical methods, with preoperative and postoperative chemotherapy. Such intervention is very complex and requires special qualifications of a neurosurgeon.
Radiation surgery and, in particular, stereotactic surgery with exposure to high ionizing doses is quite effective. The use of such a technique at early stages of neoplasm development sometimes allows to achieve prolonged remission or even complete cure of the patient.
Radiation is often combined with chemotherapy, which improves the efficacy of interventions and reduces the radiation burden. In gliomas, not all chemopreventive agents are therapeutically successful, so they are prescribed individually and the prescriptions are adjusted if necessary.
To reduce pain and lower intracranial pressure, regardless of the main treatment, symptomatic therapy is prescribed - in particular, corticosteroid drugs, analgesics, sedatives.
Medications
Corticosteroid drugs affect swelling, reduce the severity of neurologic symptoms for several days. However, due to multiple side effects and increased likelihood of adverse interactions with chemotherapy drugs, minimally effective doses of steroids are used, discontinuing them as soon as possible (e.g., after surgery).
Anticonvulsants are used systematically as a secondary preventive measure in patients who have already experienced epileptic seizures. These medications can cause serious adverse symptoms and also interact with chemotherapy drugs.
Anticoagulants are especially relevant at the postoperative stage, since the risks of thrombophlebitis formation in glioma are quite high (up to 25%).
A good effect is expected from taking antidepressant-anxiolytics. The use of Methylphenidate 10-30 mg/day in two doses often allows to optimize cognitive abilities, improve quality of life, maintain working capacity.
|
Neurological failure and signs of cerebral edema (pain in the head, disturbances of consciousness) are eliminated by corticosteroid drugs - in particular, Prednisolone or Dexamethasone. |
|
The scheme and dosage of corticosteroids is selected individually, with the practice of the minimum effective dose. At the end of the treatment course, the drugs are gradually withdrawn. |
|
Corticosteroids are taken together with gastroprotective drugs - proton pump blockers or H2-histamineBlockers. |
|
Diuretics (Furosemide, Mannitol) are prescribed for severe swelling and displacement of brain structures, as an adjunct to corticosteroid drugs. |
|
In case of convulsive seizures (including anamnesis) or epileptiform symptoms on electroencephalogram, anticonvulsant therapy is additionally prescribed. Anticonvulsants are not prescribed for prophylactic purposes. |
|
Patients with indications for chemotherapy are recommended to take anticonvulsants that do not affect liver enzyme function. Drugs of choice: Lamotrigine, Valproic acid, Levetiracetam. Should not be used: Carbamazepine, Phenobarbital. |
|
Head pain in brain gliomas is managed with corticosteroid treatment. |
|
In some cases of headaches, non-steroidal anti-inflammatory drugs or tramadol may be used. |
|
If the patient is taking non-steroidal anti-inflammatory drugs, they are discontinued a few days before surgery to minimize the chance of bleeding during surgery. |
|
In selected pain cases, narcotic analgesics - such as Fentanyl or Trimeperidine - may be recommended. |
|
To prevent pulmonary embolism from the third postoperative day, administration of low-molecular-weight heparins - in particular, Enoxaparin sodium or Nadroparin calcium - is prescribed. |
|
If the patient is on systematic anticoagulant or antiaggregant treatment, he is transferred to low-molecular-weight heparins no later than a week before surgical intervention, with their further withdrawal a day before surgery and resumption 24-48 hours after surgery. |
|
If a patient with glioma has venous thrombosis of the lower extremities, treatment with direct anticoagulants is performed. The possibility of placing a CAVA-filter is not excluded. |
Chemotherapy for malignant gliomas of the brain
The basic antitumor chemotherapy regimens for gliomas are considered to be:
- Lomustine 100 mg/m² on day one, Vincristine 1.5 mg/m² on days one and eight, Procarbazine 70 mg/m² from day eight to twenty-first, courses every six weeks.
- Lomustine 110 mg/m² every six weeks.
- Temozolomide 5/23 150 to 200 mg/m² from day one to day five, every 28 days.
- Temozolomide as part of chemoradiation treatment, 75 mg/m² each day that radiation is given.
- Temozolomide with Cisplatin or Carboplatin (80 mg/m²), and Temozolomide 150-200 mg/m² on days 1 through 5 every 4 weeks.
- Temozolomide 7/7 at 100 mg/m² on days 1-8 and 15-22 of the course, with a repeat every four weeks.
- Bevacizumab 5 to 10 mg/kg on days one and fifteen, and Irinotecan 200 mg/m² on days one and fifteen, repeated every four weeks.
- Bevacizumab 5 to 10 mg/kg on days one, fifteen, and twenty-nine, and Lomustine 90 mg/m² on day one every six weeks.
- Bevacizumab 5 to 10 mg/kg on days one and fifteen, Lomustine 40 mg on days one, eight, fifteen, and twenty-two, repeated every six weeks.
- Bevacizumab 5 to 10 mg/kg on days one and fifteen, repeated every four weeks.
Cytostatic drugs in many cases successfully inhibit the growth of tumor cells, but do not show selectivity towards healthy tissues and organs. Therefore, experts have identified a number of contraindications in which chemotherapy of glioma is impossible:
- Excessive individual sensitivity to chemopreventive agents;
- Decompensation of cardiac, renal, hepatic function;
- Depressed hematopoiesis in the bone marrow;
- Adrenal function problems.
Chemotherapy is administered with extreme caution:
- Patients with significant heart rhythm disturbances;
- With diabetes;
- For acute viral infections;
- To elderly patients;
- Patients suffering from chronic alcoholism (chronic alcohol intoxication).
The most serious side effect of chemopreventive drugs is their toxicity: cytostatics selectively interfere with the functionality of blood cells and change their composition. As a consequence, platelet and erythrocyte mass decreases and anemia develops.
Before prescribing a patient a course of chemotherapy, the doctor always takes into account the degree of toxicity of the drugs and the likely complications after their use. Chemotherapy courses are always carefully monitored by specialists and regular blood monitoring.
Possible consequences of cytostatic therapy:
- Gauntness, emaciation;
- Difficulty swallowing food, dry mucous membranes, periodontitis, dyspepsia;
- Central nervous system instability, manic-depressive disorders, seizure syndrome, asthenia;
- Deterioration of auditory function;
- Increase in blood pressure up to the development of hypertensive crisis;
- Decrease in platelets, red blood cells, white blood cells, multiple hemorrhages, internal and external bleeding;
- Renal failure;
- Allergic processes;
- Hair loss, the appearance of areas of increased pigmentation.
After courses of chemotherapy, patients have an increased risk of developing infectious diseases, and muscle and joint pain is common.
To reduce the risk of adverse post-chemotherapeutic effects, further rehabilitation measures are necessarily prescribed, the purpose of which is to restore normal blood count, stabilization of cardiovascular activity, normalization of neurological status. Sufficient psychological support is necessarily practiced.
Surgical treatment
The surgery is performed to remove the tumor focus as much as possible, which in turn should reduce intracranial pressure, reduce neurological insufficiency, and provide the necessary biomaterial for research.
- The surgery is performed in a specialized neurosurgical department or clinic whose specialists are experienced in neuro-oncological interventions.
- The surgeon performs access by plastic bone trepanation in the area of suspected glioma localization.
- If the neoplasm is located anatomically close to motor areas or pathways, or in the nuclei or along cranial nerves, intraoperative neurophysiologic monitoring is used.
- Neuronavigation systems, intraoperative fluorescence navigation with 5-aminolevulenic acid is desirable to maximize the removal of the neoplasm.
- After the intervention, a control CT or MRI (with or without contrast injection) is performed on day 1-2.
|
If surgical resection of glioma is impossible or initially recognized as inexpedient, or if lymphoma of the central nervous system is suspected, a biopsy (open, stereotactic, with navigation monitoring, etc.) is performed. |
|
Patients with cerebral gliomatosis are verified by stereotactic biopsy, since therapeutic tactics largely depend on the histologic picture. |
|
In certain situations - in elderly patients, in case of severe neurological disorders, in case of localization of glioma in the trunk and other vital parts - treatment is planned based on symptoms and imaging information after a general medical consultation. |
|
Patients with piloid astrocytoma as well as nodular forms of brainstem neoplasms and exophytic processes are recommended to undergo resection or open biopsy. |
|
Patients with diffuse pontine glioma and other diffuse neoplasms of the trunk are treated with radiation and antitumor drug therapy. Verification is not necessary in such cases. |
|
Patients with quadriplegic plate glioma undergo systematic magnetic resonance and clinical monitoring after removal of cerebral hydrocele. If the neoplasm shows signs of growth, it is removed with further irradiation. |
|
When partial resection or biopsy of a low-grade malignant glioma is performed, patients with two or more risk factors are necessarily treated with radiation and/or chemotherapy. |
|
Total resection is mandatory for patients with subependymal giant cell astrocytoma. |
|
Everolimus is prescribed for diffuse subependymal giant cell astrocytoma. |
|
Piloid astrocytoma should be removed with magnetic resonance imaging after intervention to clarify the quality of radical resection of tumor tissue. |
|
In glioblastoma, postoperative therapy should be combined (radiation + chemotherapy) with Temozolomide administration. |
|
In anaplastic astrocytoma after surgery, radiation therapy with further drug therapy is indicated. Lomustine, Temozolomide are used. |
|
Patients with anaplastic oligodendroglioma or oligoastrocytoma receive both radiation and chemotherapy (Temozolomide or PCV monotherapy) after surgery. |
|
Elderly patients with extensive high malignant glioma are irradiated in hypofractionated mode, or monotherapy with Temozolomide is performed. |
|
In case of glioma recurrence, the possibility of reoperation and subsequent treatment tactics are discussed by a consilium of specialists. The optimal regimen for recurrences: reoperation + systemic chemotherapy + repeated radiation exposure + palliative measures. If there are localized minor areas of recurrent tumor growth, radiosurgery may be used. |
|
The drugs of choice for recurrent glioma growth are Temozolomide and Bevacizumab. |
|
Recurrence of highly malignant oligodendrogliomas and anaplastic astrocytomas is an indication for Temozolomide treatment. |
|
Pleomorphic xanthoastrocytoma is removed without mandatory adjuvant chemotherapy. |
One of the peculiarities of gliomas is the difficulty in their treatment and removal. The surgeon aims to remove the tissues of the neoplasm as completely as possible, to achieve compensation of the condition. Many patients are able to improve the quality of life and prolong it, but for highly malignant tumors the prognosis remains unfavorable: there is an increased likelihood of re-growth of the pathological focus.
Nutrition for glioma of the brain
Diet for patients with malignant tumors - an important point, which, unfortunately, many people do not pay much attention to. Meanwhile, thanks to changes in diet, it is possible to slow down the development of glioma and strengthen and shaken immunity.
Major areas of dietary change:
- Normalization of metabolic processes, strengthening immune protection;
- Detoxification of the body;
- Optimization of energy potential;
- Ensuring normal functioning of all organs and systems of the body during such a difficult period for them.
Rational and balanced diet is necessary, as patients with early stages of low malignant neoplasms, and patients with the last stage of glioblastoma. Carefully selected diet contributes to the improvement of general well-being, recovery of damaged tissues, which is especially important against the background of cytostatic and radiation treatment. The balance of nutritional components and proper metabolic processes prevent the formation of infectious foci, block inflammatory reactions, prevent exhaustion of the body.
The following foods and beverages are recommended for brain glioma:
- Red, yellow and orange colored fruits and vegetables (tomatoes, peaches, apricots, carrots, beets, citrus fruits) containing carotenoids, which protect healthy cells from the negative effects of radiation therapy;
- Cabbage (cauliflower, broccoli, Brussels sprouts), radish, mustard, and other plant products that contain indole - an active substance that neutralizes adverse toxic and chemical factors;
- Greens (dill, parsley, young dandelion and nettle leaves, rhubarb, arugula, spinach), green peas and asparagus, asparagus beans, and algae (seaweed, spirulina, chlorella);
- Green tea;
- Garlic, onion, pineapple, which have anti-tumor and detoxification ability;
- Bran, cereals, whole grain bread, sprouted sprouts of legumes, grains and seeds;
- Dark grapes, raspberries, strawberries and strawberries, blueberries, blackberries, pomegranate, currants, blackcurrants, rowanberries, blueberries, sea buckthorn, cherries and other berries that contain natural antioxidants that reduce the negative effects of free radicals, viruses and carcinogens;
- Low-fat dairy products.
You should not burden the digestive system and the whole body with heavy and fatty foods. It is useful to use freshly squeezed homemade juices, smoothies, morsels. Sources of omega-3 fatty acids, such as fish oil, flaxseed oil or flax seeds, should be added to dishes.
It is better to avoid sugar and sweets altogether. But a spoonful of honey with a cup of water will not hurt: bee products have a pronounced anti-inflammatory, antioxidant and antitumor effect. The only contraindication to the use of honey is an allergy to the product.
From the diet should be excluded:
- Meat, lard, offal;
- Butter, fatty dairy products;
- Smoked meat, sausages, canned meat and fish;
- Alcohol in any form;
- Sweets, pastries, cakes and pastries, candies and chocolates;
- Convenience foods, fast food, snacks;
- Fried foods.
You should consume enough vegetables, greens, fruits, and clean drinking water on a daily basis.
During chemotherapy and for some time after it, you should drink homemade vegetable and fruit juices, eat homemade low-fat cottage cheese, milk and cheese. It is important to drink plenty of fluids, brush your teeth and rinse your mouth often (about 4 times a day).
Optimal meals for brain glioma patients:
- Vegetable casseroles;
- Side dishes and soups made of cereals (preferably buckwheat, oatmeal, rice, couscous, bulgur);
- Steamed cheesecakes, puddings, casseroles;
- Stewed and baked vegetables;
- Stews, vegetable soups, first and second dishes from legumes (including soy), pâtés and soufflés;
- Smoothies, green tea, compotes and morsels.
Prevention
If a person leads a healthy lifestyle, and among his relatives there were no cases of cancer pathology, he has every chance not to get brain glioma. There is no specific prevention of such tumors, so the main preventive points are considered to be proper nutrition, physical activity, avoidance of bad habits, absence of occupational and household hazards.
Specialists give a number of simple, yet effective recommendations:
- Drink more pure water, avoid sweetened sodas, packaged juices, energy drinks, and alcohol.
- Avoid occupational and household hazards: less contact with chemicals, corrosive solutions and liquids.
- Try to prepare food by boiling, stewing, baking, but not frying. Give preference to healthy, quality homemade foods.
- A large proportion of your diet should be plant foods, including greens, regardless of the time of year.
- Another negative factor is being overweight, which should be gotten rid of. Weight control is very important for the health of the whole body.
- Vegetable oils should always be preferred over butter and lard.
- If possible, it is desirable to give preference to environmentally friendly products, meat without hormones, vegetables and fruits without nitrates and pesticides. It is better to avoid red meat altogether.
- Do not take multivitamin preparations without indications and in large quantities. Do not take any medication without a doctor's prescription: self-medication is often very, very dangerous.
- If suspicious symptoms appear, it is necessary to visit a doctor, without waiting for the aggravation of the situation, the development of adverse effects and complications.
- Sweets and foods with a high glycemic index are an undesirable component of the diet.
- The earlier a person goes to the doctors, the better his chances of cure (and this applies to almost any disease, including glioma of the brain).
To prevent the formation of oncopathology, you need enough time to sleep and rest, avoid excessive consumption of alcoholic beverages, give preference to high-quality natural food, reduce the use of gadgets (in particular, cell phones).
Tumor diseases often occur in elderly and old people. Therefore, it is important to monitor your own health from a young age and not to provoke pathological processes by unhealthy lifestyle and unhealthy habits.
The exact root causes of oncology have not yet been clarified. However, a certain role, of course, play unfavorable occupational and environmental conditions, exposure to ionizing and electromagnetic radiation, hormonal changes. Do not stay long and regularly under the sun, allow sudden changes in ambient temperature, overheat in the bath or sauna, often take hot baths or showers.
Another question: how to prevent a recurrence of brain glioma after its successful treatment? Recurrence of neoplasm growth is a complex and, unfortunately, frequent complication, which is difficult to predict in advance. Patients can be recommended to undergo regular preventive examinations and checkups, visit an oncologist and attending physician at least twice a year, lead a healthy lifestyle, eat healthy and natural food, practice moderate physical activity. Another condition is love of life, healthy optimism, positive attitude to success under any circumstances. This also includes a friendly atmosphere in the family and at work, patience and unconditional support from close people.
Forecast
The condition of the brain and the characteristics of the glioma at the time of its detection influence the survival rate as much as the treatment administered. A satisfactory general health of the patient and his age improve the prognosis (the prognosis is more optimistic in young patients). An important indicator is the histologic picture of the neoplasm. Thus, low-grade gliomas have a better prognosis than anaplastic gliomas and, even more so, glioblastomas (the most unfavorable tumor processes). Astrocytomas have a worse prognosis than oligodendrogliomas.
Malignant astrocytomas respond poorly to therapy and have a relatively low survival rate of six to five years. At the same time, life expectancy in low-grade gliomas is estimated at 1-10 years.
Malignant astrocytomas are essentially incurable. The direction of treatment usually involves reducing neurological manifestations (including cognitive dysfunction) and increasing life expectancy while maintaining the highest possible quality of life. Symptomatic therapy is attracted against the background of rehabilitation measures. The work of a psychologist is also important.
Over the past decade, scientists have made some progress in understanding the nature of brain tumors and how to treat them. Much more should be done to optimize the prognosis of the disease. The primary task of specialists today is the following: brain glioma should have several schemes for effective elimination of the problem at once, both at early and subsequent stages of development.

