Medical expert of the article
New publications
Diagnosis of osteoarthritis: magnetic resonance imaging
Last reviewed: 04.07.2025

All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.
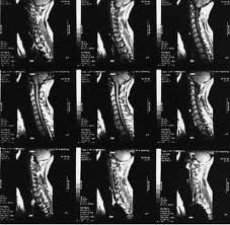
Magnetic resonance imaging (MRI) has become one of the leading methods for non-invasive diagnostics of osteoarthritis in recent years. Since the 1970s, when the principles of magnetic resonance (MR) were first used to study the human body, this method of medical imaging has changed dramatically and continues to evolve rapidly.
Technical equipment and software are being improved, image acquisition methods are being developed, and MR contrast agents are being developed. This allows new areas of application for MRI to be constantly found. If at first its application was limited to studies of the central nervous system, now MRI is successfully used in almost all areas of medicine.
In 1946, groups of researchers from Stanford and Harvard Universities independently discovered a phenomenon called nuclear magnetic resonance (NMR). Its essence was that the nuclei of some atoms, being in a magnetic field, under the influence of an external electromagnetic field are capable of absorbing energy and then emitting it in the form of a radio signal. For this discovery, F. Bloch and E. Parmel were awarded the Nobel Prize in 1952. The new phenomenon was soon used for spectral analysis of biological structures (NMR spectroscopy). In 1973, Paul Rautenburg first demonstrated the possibility of obtaining an image using NMR signals. This is how NMR tomography appeared. The first NMR tomograms of the internal organs of a living person were demonstrated in 1982 at the International Congress of Radiologists in Paris.
Two clarifications should be given. Despite the fact that the method is based on the NMR phenomenon, it is called magnetic resonance (MR), omitting the word "nuclear". This is done so that patients do not have thoughts about radioactivity associated with the decay of atomic nuclei. And the second circumstance: MR tomographs are not accidentally "tuned" to protons, i.e., hydrogen nuclei. There is a lot of this element in tissues, and its nuclei have the greatest magnetic moment among all atomic nuclei, which determines a fairly high level of MR signal.
If in 1983 there were only a few devices suitable for clinical research in the world, then by the beginning of 1996 there were about 10,000 tomographs in operation worldwide. Every year 1000 new devices are introduced into practice. More than 90% of the park of MR-tomographs are models with superconducting magnets (0.5-1.5 T). It is interesting to note that if in the mid-80s the companies - manufacturers of MR-tomographs were guided by the principle "the higher the field, the better", focusing on models with a field of 1.5 T and higher, then by the end of the 80s it became clear that in most areas of application they do not have significant advantages over models with an average field strength. Therefore, the main manufacturers of MR tomographs (General Electric, Siemens, Philips, Toshiba, Picker, Bruker, etc.) are currently paying much attention to the production of models with medium and even low fields, which differ from high-field systems in their compactness and economy with satisfactory image quality and significantly lower cost. High-field systems are used primarily in research centers for MR spectroscopy.
Principle of the MRI method
The main components of an MRI scanner are: a super-strong magnet, a radio transmitter, a receiving radio frequency coil, a computer, and a control panel. Most devices have a magnetic field with a magnetic moment parallel to the long axis of the human body. Magnetic field strength is measured in teslas (T). For clinical MRI, fields with a strength of 0.2-1.5 T are used.
When a patient is placed in a strong magnetic field, all protons, which are magnetic dipoles, turn in the direction of the external field (like a compass needle oriented toward the Earth's magnetic field). In addition, the magnetic axes of each proton begin to rotate around the direction of the external magnetic field. This specific rotational motion is called procession, and its frequency is called resonant frequency. When short electromagnetic radiofrequency pulses are passed through the patient's body, the magnetic field of the radio waves causes the magnetic moments of all protons to rotate around the magnetic moment of the external field. For this to happen, the frequency of the radio waves must be equal to the resonant frequency of the protons. This phenomenon is called magnetic resonance. To change the orientation of the magnetic protons, the magnetic fields of the protons and radio waves must resonate, i.e. have the same frequency.
A net magnetic moment is created in the patient's tissues: the tissues are magnetized and their magnetism is oriented strictly parallel to the external magnetic field. The magnetism is proportional to the number of protons per unit volume of tissue. The enormous number of protons (hydrogen nuclei) contained in most tissues means that the net magnetic moment is large enough to induce an electric current in a receiving coil located outside the patient. These induced MR signals are used to reconstruct the MR image.
The process of transition of the electrons of the nucleus from the excited state to the equilibrium state is called the spin-lattice relaxation process or longitudinal relaxation. It is characterized by T1 - the spin-lattice relaxation time - the time required to transfer 63% of the nuclei to the equilibrium state after their excitation by a 90° pulse. T2 - the spin-spin relaxation time is also distinguished.
There are several methods for obtaining MR tomograms. They differ in the order and nature of radiofrequency pulse generation and methods of MR signal analysis. The two most widely used methods are spin-lattice and spin-echo. Spin-lattice mainly analyzes the T1 relaxation time. Different tissues (gray and white matter of the brain, cerebrospinal fluid, tumor tissue, cartilage, muscles, etc.) contain protons with different T1 relaxation times. The intensity of the MR signal is related to the duration of T1: the shorter the T1, the more intense the MR signal and the brighter the given area of the image appears on the TV monitor. Fatty tissue is white on MR tomograms, followed by the brain and spinal cord, dense internal organs, vascular walls and muscles in descending order of MR signal intensity. Air, bones and calcifications practically do not produce an MR signal and are therefore displayed in black. These T1 relaxation time relationships create the prerequisites for visualizing normal and altered tissues on MRI scans.
In another method of MRI, called spin-echo, a series of radiofrequency pulses are directed at the patient, rotating the precessing protons by 90°. After the pulses stop, the response MRI signals are recorded. However, the intensity of the response signal is related differently to the duration of T2: the shorter the T2, the weaker the signal and, consequently, the lower the brightness of the glow on the TV monitor screen. Thus, the final MRI picture using the T2 method is the opposite of that using the T1 method (as a negative is the opposite of a positive).
MRI tomograms display soft tissues better than CT scans: muscles, fat layers, cartilage, and blood vessels. Some devices can produce images of blood vessels without injecting a contrast agent (MRI angiography). Due to the low water content in bone tissue, the latter does not create a shielding effect, as in X-ray CT scanning, i.e., it does not interfere with the image of, for example, the spinal cord, intervertebral discs, etc. Of course, hydrogen nuclei are not only contained in water, but in bone tissue they are fixed in very large molecules and dense structures and do not interfere with MRI.
Advantages and disadvantages of MRI
The main advantages of MRI include non-invasiveness, harmlessness (no radiation exposure), three-dimensional nature of image acquisition, natural contrast from moving blood, no artifacts from bone tissue, high differentiation of soft tissues, the ability to perform MP spectroscopy for in vivo tissue metabolism studies. MRI allows obtaining images of thin layers of the human body in any section - in the frontal, sagittal, axial and oblique planes. It is possible to reconstruct volumetric images of organs, synchronize the acquisition of tomograms with the teeth of the electrocardiogram.
The main disadvantages usually include the relatively long time required to obtain images (usually minutes), which leads to the appearance of artifacts from respiratory movements (this especially reduces the effectiveness of lung examination), arrhythmias (in cardiac examination), the inability to reliably detect stones, calcifications, some types of bone pathology, the high cost of equipment and its operation, special requirements for the premises in which the devices are located (shielding from interference), the inability to examine patients with claustrophobia, artificial pacemakers, large metal implants made of non-medical metals.
 [ 8 ], [ 9 ], [ 10 ], [ 11 ], [ 12 ], [ 13 ], [ 14 ], [ 15 ]
[ 8 ], [ 9 ], [ 10 ], [ 11 ], [ 12 ], [ 13 ], [ 14 ], [ 15 ]
Contrast agents for MRI
At the beginning of MRI use, it was believed that the natural contrast between different tissues eliminated the need for contrast agents. It was soon discovered that the difference in signals between different tissues, i.e. the contrast of the MR image, could be significantly improved by contrast agents. When the first MR contrast agent (containing paramagnetic gadolinium ions) became commercially available, the diagnostic information content of MRI increased significantly. The essence of using MR contrast agents is to change the magnetic parameters of tissue and organ protons, i.e. to change the relaxation time (TR) of T1 and T2 protons. Today, there are several classifications of MR contrast agents (or rather contrast agents - CA).
According to the predominant effect on the relaxation time, MR-KA is divided into:
- T1-CA, which shorten T1 and thereby increase the intensity of the tissue MP signal. They are also called positive CA.
- T2-CAs that shorten T2, reducing the intensity of the MR signal. These are negative CAs.
Depending on their magnetic properties, MR-CA are divided into paramagnetic and superparamagnetic:
 [ 16 ], [ 17 ], [ 18 ], [ 19 ], [ 20 ]
[ 16 ], [ 17 ], [ 18 ], [ 19 ], [ 20 ]
Paramagnetic contrast agents
Paramagnetic properties are possessed by atoms with one or more unpaired electrons. These are magnetic ions of gadolinium (Gd), chromium, nickel, iron, and manganese. Gadolinium compounds have received the widest clinical application. The contrast effect of gadolinium is due to the shortening of the relaxation time T1 and T2. In low doses, the effect on T1 predominates, increasing the signal intensity. In high doses, the effect on T2 predominates, reducing the signal intensity. Paramagnets are now most widely used in clinical diagnostic practice.
Superparamagnetic contrast agents
The dominant effect of superparamagnetic iron oxide is the shortening of T2 relaxation. With increasing dose, there is a decrease in signal intensity. Ferromagnetic CAs, which include ferromagnetic iron oxides structurally similar to magnetite ferrite (Fe 2+ OFe 23+ O 3 ), can also be included in this group of CAs.
The following classification is based on the pharmacokinetics of CA (Sergeev P.V. et al., 1995):
- extracellular (tissue-non-specific);
- gastrointestinal;
- organotropic (tissue-specific);
- macromolecular, which are used to determine the vascular space.
In Ukraine, four MR-CA are known, which are extracellular water-soluble paramagnetic CA, of which gadodiamide and gadopentetic acid are widely used. The remaining groups of CA (2-4) are undergoing clinical trials abroad.
Extracellular water-soluble MR-CA
International name |
Chemical formula |
Structure |
Gadopentetic acid |
Gadolinium dimeglumine diethylenetriamine penta-acetate ((NMG)2Gd-DTPA) |
Linear, ionic |
Gadoteric acid |
(NMG)Gd-DOTA |
Cyclic, ionic |
Gadodiamide |
Gadolinium diethylenetriamine pentaacetate-bis-methylamide (Gd-DTPA-BMA) |
Linear, non-ionic |
Gadoteridol |
Gd-HP-D03A |
Cyclic, non-ionic |
Extracellular CA are administered intravenously, 98% of them are excreted by the kidneys, do not penetrate the blood-brain barrier, have low toxicity, and belong to the group of paramagnetic substances.
Contraindications to MRI
Absolute contraindications include conditions in which the examination poses a threat to the life of patients. For example, the presence of implants that are activated electronically, magnetically or mechanically - these are primarily artificial pacemakers. Exposure to radiofrequency radiation from an MRI scanner may disrupt the functioning of a pacemaker operating in the request system, since changes in magnetic fields may imitate cardiac activity. Magnetic attraction may also cause the pacemaker to shift in its socket and move the electrodes. In addition, the magnetic field creates obstacles to the operation of ferromagnetic or electronic middle ear implants. The presence of artificial heart valves is dangerous and is an absolute contraindication only when examined on MRI scanners with high fields, and if damage to the valve is clinically suspected. Absolute contraindications to the examination also include the presence of small metal surgical implants (hemostatic clips) in the central nervous system, since their displacement due to magnetic attraction threatens bleeding. Their presence in other parts of the body poses less of a threat, since after treatment, fibrosis and encapsulation of the clamps help to keep them stable. However, in addition to the potential danger, the presence of metallic implants with magnetic properties in any case causes artifacts that create difficulties in interpreting the results of the study.
Contraindications to MRI
Absolute: |
Relative: |
Pacemakers |
Other stimulants (insulin pumps, nerve stimulators) |
Ferromagnetic or electronic middle ear implants |
Non-ferromagnetic inner ear implants, heart valve prostheses (in high fields, if dysfunction is suspected) |
Hemostatic clips of cerebral vessels |
Hemostatic clips in other locations, decompensated heart failure, pregnancy, claustrophobia, need for physiological monitoring |
Relative contraindications, in addition to those listed above, include decompensated heart failure, the need for physiological monitoring (mechanical ventilation, electric infusion pumps). Claustrophobia is an obstacle to the study in 1-4% of cases. It can be overcome, on the one hand, by using devices with open magnets, on the other - by a detailed explanation of the device and the course of the examination. There is no evidence of a damaging effect of MRI on the embryo or fetus, but it is recommended to avoid MRI in the first trimester of pregnancy. The use of MRI during pregnancy is indicated in cases where other non-ionizing diagnostic imaging methods do not provide satisfactory information. MRI examination requires more patient participation than computed tomography, since patient movements during the examination have a much greater effect on the quality of the images, so the examination of patients with acute pathology, impaired consciousness, spastic conditions, dementia, as well as children is often difficult.

