Medical expert of the article
New publications
Arteriovenous malformation
Last reviewed: 04.07.2025

All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.
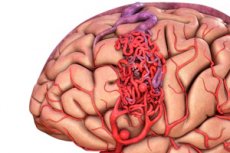
Arteriovenous malformation is a congenital defect in the development of blood vessels, which is characterized by the presence of an abnormal network of arteriovenous anastomoses. Most often, arteriovenous malformations are located in the posterior cranial fossa and have a fairly typical structure - one or two true arteries, a tangle of AVMs and one draining vein.
 [ 1 ]
[ 1 ]
Causes arteriovenous malformation
The causes that may underlie the development of AVM are not fully understood, but it is believed that they may be related to genetic and environmental factors. Here are some of the possible causes that may be associated with the development of AVM:
- Genetic factors: Studies have shown that some patients with AVMs have a family history of the condition. Genetic mutations may play a role in the formation of the vascular abnormality.
- Congenital defects: Congenital vascular anomalies can develop during embryonic development. These may be due to errors in the development of the brain's vascular tissues.
- Environmental factors: Some studies have shown a link between AVMs and certain environmental factors, such as radiation or toxic exposures during pregnancy. However, these links are not always confirmed and do not explain all cases of AVMs.
- Regional factors: In some cases, AVM may be associated with certain geographic areas or ethnic groups, but these associations require further study.
In addition to these factors that may influence the development of AVM, it is important to note that this is a congenital condition and is usually detected at birth or in early childhood, although symptoms may appear later in life. Further research is needed to accurately understand the causes and mechanisms of AVM development.
Pathogenesis
The most dangerous is the rupture of the malformation walls, which is accompanied by spontaneous intracranial hemorrhage. This occurs due to the fact that mixed blood circulates in the malformation vessels under pressure close to arterial. And naturally, high pressure leads to stretching of the degeneratively changed vessels, an increase in their volume and thinning of the wall. Ultimately, a rupture occurs in the thinnest place. According to statistical data, this occurs in 42-60% of patients with AVM. Mortality at the first AVM rupture reaches 12-15%. In the rest, bleeding can be repeated, without any periodicity. We observed a patient who had eleven spontaneous intracranial hemorrhages over 8 years. Such a relatively "benign" course of AVM rupture compared to rupture of arterial aneurysms is explained by the peculiarities of hemodynamic disorders that occur after the rupture. It is known that the rupture of an arterial aneurysm most often leads to subarachnoid hemorrhage (SAH) and the development of angiospasm, which in the first minutes is protective in nature, helping to quickly stop bleeding, but subsequently poses a major danger to the patient's life.
It is the angiospasm that leads to cerebral ischemia and edema that determines the severity of the patient's condition and the prognosis. In contrast, angiospasm of the afferent arteries of the AVM, on the contrary, improves the blood supply to the brain due to a decrease in arteriovenous discharge. When an AVM ruptures, intracerebral and subdural hematomas are more often formed. The breakthrough of blood into the subarachnoid cisterns is secondary. Bleeding from the ruptured wall of the AVM stops faster, since the blood pressure in it is lower than in the main arteries and the wall is more susceptible to compression by the spilled blood. Naturally, this does not always end well for the patient. The most dangerous AVM ruptures are near the ventricles of the brain, in the subcortical ganglia and in the brainstem. Angiospasm of the afferent arteries in this situation helps stop the bleeding.
The determining factor in the pathogenesis of AVM rupture is the volume of spilled blood and the localization of the hematoma. Hemispheric intracerebral hematomas, even with a volume of up to 60 cm 3, proceed relatively favorably. They can cause severe focal neurological disorders, but rarely lead to severe vital disorders. A hematoma rupture into the ventricles of the brain significantly worsens the prognosis. On the one hand, blood, irritating the ependyma of the ventricles, increases cerebrospinal fluid production, on the other hand, affecting the bottom of the ventricle, leads to severe disorders of the functions of vital centers located in the hypothalamus. The spread of blood throughout the ventricular system leads to tamponade of the latter, which in itself is incompatible with life.
Blood that has penetrated into the subarachnoid cisterns also disrupts cerebrospinal fluid circulation, making it difficult for cerebrospinal fluid to reach the blood-blocked pacchion granulations. As a result, cerebrospinal fluid resorption slows down and acute cerebrospinal fluid hypertension may develop, followed by internal and external hydrocephalus. As a result of the breakdown of the formed elements of the spilled blood, a large number of toxic substances are formed, most of which have a vasoactive effect. On the one hand, this leads to vasoconstriction of small pial arteries, and on the other, it increases capillary permeability. Blood breakdown products also affect nerve cells, changing their biochemical processes and disrupting the permeability of cell membranes. First of all, the function of the potassium-sodium pump changes and potassium begins to leave the cell, and the sodium cation, which is four times more hydrophilic than potassium, rushes to take its place.
This leads first to intracellular edema in the area around the hemorrhage, and then to swelling of the cells. Hypoxia also contributes to the development of edema, which inevitably joins in due to compression of the brain vessels by the hematoma and increased cerebrospinal fluid pressure, which has already been said. Dysfunction of the diencephalic parts of the brain and, above all, regulation of the water-electrolyte balance leads to fluid retention in the body, loss of potassium, which also increases the edematous reaction of the brain. The pathogenesis of AVM rupture is not limited to cerebral disorders. No less dangerous are extracerebral complications. First of all, this is cerebrocardial syndrome, which on the electrocardiogram can simulate acute coronary insufficiency.
Quite quickly, patients with intracerebral hemorrhages develop pneumonia and respiratory failure. Moreover, the bacterial flora plays a secondary role. The primary effect is the central influence on the lungs, consisting of widespread bronchospasm, increased production of sputum and mucus, ischemia of the pulmonary parenchyma due to widespread spasm of small pulmonary arteries, which quickly leads to dystrophic disorders, desquamation of the alveolar epithelium, and a decrease in the gas exchange function of the lungs.
If this is accompanied by suppression of the cough reflex, bulbar type of respiratory failure, then a serious threat to the patient's life arises. In most cases, the purulent trachyobronchitis that follows is poorly amenable to antibacterial therapy and aggravates respiratory failure, which immediately affects the increase in cerebral hypoxia. Thus, the violation of external respiration, even with relative compensation of cerebral disorders, can lead to a fatal outcome. Often, patients after a coma regain consciousness, but then die from increasing respiratory failure and hypoxic cerebral edema.
Dystrophic changes develop rapidly not only in the lungs, but also in the liver, gastrointestinal tract, adrenal glands and kidneys. Urinary tract infection and bedsores, which develop rapidly in the absence of good patient care, pose a threat to the patient's life. But these complications can be avoided if doctors remember them and know how to combat them.
In summing up the examination of the pathogenesis of AVM rupture, it should be emphasized that mortality in such intracranial hemorrhages is lower than in rupture of arterial aneurysms and hypertensive hemorrhagic strokes, although it reaches 12-15%. AVMs are characterized by repeated, sometimes multiple hemorrhages with varying periodicity, which is impossible to predict. In case of an unfavorable course of the posthemorrhagic period, the listed pathogenetic mechanisms can lead to a fatal outcome.
Symptoms arteriovenous malformation
Hemorrhagic type of the disease (50-70% of cases). This type is characterized by the presence of arterial hypertension in the patient, a small size of the malformation node, its drainage into deep veins, arteriovenous malformation of the posterior cranial fossa is quite common.
Hemorrhagic type in 50% of cases is the first symptom of manifestation of arteriovenous malformation, causes detailed result and 10-15% and disability of 20-30% of patients (N. Martin et al.). Annual risk of hemorrhage in patients with arteriovenous malformation is 1.5-3%. Risk of repeated hemorrhage during the first year reaches 8% and increases with age. Bleeding from arteriovenous malformation is the cause of 5-12% of all maternal mortality and 23% of all intracranial hemorrhages in pregnant women. Picture of subarachnoid hemorrhage is observed in 52% of patients. In 17% of patients complicated forms of hemorrhage occur: formation of intracerebral (38%), subdural (2%) and mixed (13%) hematomas, hemotamponade of ventricles develops in 47%.
The torpid type of course is typical for patients with large arteriovenous malformations localized in the cortex. The blood supply to the arteriovenous malformation is provided by branches of the middle cerebral artery.
The most characteristic symptoms of the torpid type of course are convulsive syndrome (in 26-27% of patients with arteriovenous malformation), cluster headaches, and progressive neurological deficit, as with brain tumors.
Variants of clinical manifestations of arteriovenous malformations
As already mentioned, the most common first clinical manifestation of AVM is spontaneous intracranial hemorrhage (40-60% of patients). It often occurs without any precursors, in the midst of complete health. Provoking moments can be physical exertion, stressful situation, neuropsychic tension, taking large doses of alcohol, etc. At the moment of AVM rupture, patients feel a sudden sharp headache, like a blow or rupture. The pain quickly increases, causing dizziness, nausea and vomiting.

Loss of consciousness may occur within a few minutes. In rare cases, headache may be mild, patients do not lose consciousness, but feel their limbs weakening and going numb (usually contralateral to the hemorrhage), and speech is impaired. In 15% of cases, hemorrhage manifests itself as a full-blown epileptic seizure, after which patients may remain in a comatose state.
To determine the severity of hemorrhage from AVM, the Hunt-Hess scale given above can be used as a basis with some adjustments. Due to the fact that hemorrhages from AVM can have very different symptoms, focal neurological symptoms can prevail over general cerebral symptoms. Therefore, patients with consciousness levels at I or II levels of the scale can have severe focal neurological disorders (hemiparesis, hemihypesthesia, aphasia, hemianopsia). Unlike aneurysmal hemorrhages, AVM rupture is determined not by the severity and prevalence of angiospasm, but by the volume and localization of the intracerebral hematoma.
Meningeal syndrome develops after several hours and its severity may vary. Blood pressure usually increases, but not as sharply and not for as long as with ruptured arterial aneurysms. Usually, this increase does not exceed 30-40 mm Hg. On the second or third day, hyperthermia of central genesis appears. The condition of patients naturally worsens as cerebral edema increases and the breakdown of the spilled blood intensifies. This continues for up to 4-5 days. With a favorable course, after stabilization on the 6-8th day, the condition of patients begins to improve. The dynamics of focal symptoms depends on the localization and size of the hematoma.
In case of hemorrhage in functionally important areas of the brain or destruction of motor conductors, symptoms of loss appear immediately and persist for a long time without any dynamics. If symptoms of loss do not appear immediately, but increase in parallel with cerebral edema, one can expect the deficit to be restored in 2-3 weeks, when the edema completely regresses.
The clinical picture of an AVM rupture is quite diverse and depends on many factors, the main ones being: the volume and location of the hemorrhage, the severity of the cerebral edema reaction, and the degree of involvement of the brain stem structures in the process.
Arteriovenous malformations can manifest themselves as epileptiform seizures (30-40%). The cause of their development can be hemocirculatory disorders in neighboring areas of the brain due to the steal phenomenon. In addition, the malformation itself can irritate the cerebral cortex, generating epileptic discharges. And we have already talked about certain types of AVM, around which gliosis of the brain tissue develops, which is also often manifested by epileptic seizures.
An epileptic syndrome caused by the presence of an AVM is characterized by its causeless occurrence in adulthood, often in the complete absence of a provoking factor. Seizures can be generalized or focal. The presence of a clear focal component in an epileptic seizure in the absence of general cerebral symptoms should prompt the idea of a possible AVM. Even generalized seizures, if they begin with convulsions mainly in the same limbs with a forced turn of the head and eyes to one side or another, are often a manifestation of an AVM. Less often, patients experience minor seizures such as absences or twilight consciousness. The frequency and periodicity of epileptic seizures can vary: from isolated to recurring.
Forms
V.V. Lebedev and co-authors identified three variants of cerebrocardial syndrome based on ECG data:
- Type I - violation of the functions of automatism and excitability (sinus tachycardia or bradycardia, arrhythmia, atrial fibrillation);
- Type II - changes in repolarization processes, transient changes in the final phase of the ventricular complex according to the type of ischemia, myocardial damage with changes in the T wave and the position of the ST segment;
- Type III - conduction function disorder (block, signs of increased load on the right heart). These ECG changes can be combined and their severity correlates with the severity of the general condition of the patients.
Complications and consequences
An arteriovenous malformation (AVM) is a congenital vascular anomaly in which arteries and veins are connected without an intervening capillary layer. Complications and consequences of AVMs can be serious and depend on the size, location, and characteristics of the specific malformation. Some of these include:
- Stroke: One of the most serious complications of AVMs is the risk of stroke. Malformations can create abnormal pathways for blood flow, which can lead to bleeding in the brain, causing a stroke. Stroke can have varying degrees of severity and leave residual neurological deficits.
- Epilepsy: AVMs can cause epileptic seizures in some patients, especially if the malformation is located in certain areas of the brain.
- Hemorrhage: Malformations can be unpredictable and cause bleeding in the brain. This can be a life-threatening complication and lead to serious consequences.
- Hydrocephalus: If the AVM is located near the ventricles of the brain, it can cause hydrocephalus, which can lead to the accumulation of extra fluid in the brain and increased intracranial pressure.
- Neurological deficits: An AVM can damage surrounding brain tissue and cause a variety of neurological deficits, including paralysis, sensory disturbances, and deficits in speech and motor coordination.
- Pain and headaches: Patients with AVM may experience chronic pain and headaches related to the malformation.
- Psychological effects: Complications from AVMs can have a significant impact on patients' psychological well-being, including anxiety, depression, and stress.
- Lifestyle restrictions: Once an AVM is detected, patients may require lifestyle changes and risk management recommendations, including limiting physical activity and certain activities.
Diagnostics arteriovenous malformation
Diagnosis of an arteriovenous malformation (AVM) typically involves a variety of imaging tests to confirm the presence and evaluate the characteristics of the malformation. The main methods used to diagnose an AVM are:
- Magnetic resonance angiography (MRA): MRA is one of the main methods for diagnosing AVMs. It is a non-invasive test that visualizes the structure of blood vessels and blood flow in the brain using magnetic fields and radio waves. MRA can determine the location, size, and shape of the AVM.
- Digital Subtraction Angiography (DSA): This is a more invasive procedure that involves injecting a contrast agent directly into the vessels through a catheter and using X-rays to produce high-quality images of the brain vessels. DSA allows for a more detailed examination of the AVM structure and blood flow patterns.
- Computed tomography (CT): CT can be used to detect AVMs and evaluate possible complications, such as bleeding. If needed, a contrast agent may be used to improve visualization.
- Vascular duplex scanning (duplex ultrasound): This technique may be useful for examining the vessels of the neck and head and identifying blood flow disturbances associated with AVMs.
- Magnetic resonance spectroscopy (MRS): MRS can provide information about the chemical composition of tissues in the area of the AVM and reveal signs of metabolic changes.
- Echoencephalography: This is an ultrasound test that can be used to evaluate blood flow and brain structure.
- Computed tomography angiography (CTA): CTA combines CT and angiography to produce three-dimensional images of the blood vessels in the brain.
Once an AVM is diagnosed, it is important to perform a detailed assessment of the malformation’s characteristics, such as its size, shape, and severity. This will help determine whether treatment is needed and the best treatment option, including surgical removal, embolization, radiation therapy, or observation. The decision to treat an AVM should be made collaboratively between the patient and the healthcare team, taking into account the individual circumstances of each case.
Differential diagnosis
Differential diagnosis of arteriovenous malformations (AVMs) can be an important step in the evaluation of a patient with vascular changes in the brain. AVMs are vascular abnormalities in which arteries and veins are connected without an intervening capillary layer. They can cause a variety of symptoms and conditions, and differentiating them from other vascular disorders is important for proper treatment. Some conditions that may require differential diagnosis with AVMs include:
- Stroke: Strokes can mimic the symptoms of an AVM, especially if the stroke is caused by vascular changes. The distinction may require computed tomography (CT) or magnetic resonance imaging (MRI) of the brain to visualize the vascular changes.
- Hematoma: Hematomas, such as a subdural or epidural hematoma, can mimic AVM symptoms, especially if accompanied by headache and neurologic symptoms. A CT or MRI scan may help determine the cause of the symptoms.
- Brain tumors: Brain tumors can cause a variety of symptoms that may be similar to those of an AVM. Diagnosis may include MRI with contrast and other imaging studies.
- Migraine: Migraines with aura can mimic AVM symptoms, such as visual disturbances and dizziness. History and additional testing can help differentiate the two.
- Cerebral vasculitis: Vasculitis can cause inflammation of blood vessels and vascular abnormalities that may mimic the symptoms of an AVM. Biopsy or angiography may be used to diagnose vasculitis.
- Venous thrombosis: Venous thromboses can mimic the symptoms of AVM, especially if there is a disruption of venous outflow from the brain. Additional studies may help in identifying thrombosis.
For accurate differential diagnosis of AVMs, the investigation includes an extensive clinical examination, neuroeducational methods (CT, MRI, angiography), sometimes biopsy and other specialized procedures depending on the specific symptoms and circumstances.
Who to contact?
Treatment arteriovenous malformation
Treatment for an arteriovenous malformation (AVM) depends on its size, location, symptoms, and potential complications. In some cases, an AVM may be small and asymptomatic, and may not require active treatment. However, if there are symptoms or a risk of bleeding, treatment may be necessary. Some of the treatments for AVMs include:
- Surgery: Surgical removal of the AVM may be considered in cases where the malformation is in an accessible location and is of low complexity. Surgical removal can help prevent the risk of bleeding and reduce symptoms. It is a complex procedure and may carry risks such as damage to surrounding tissue and nerves.
Open (transcranial) interventions:
- Stage I - coagulation of afferents;
- Stage II - isolation of the core of the arteriovenous malformation;
- Stage III - ligation and coagulation of the efferent and removal of the arteriovenous malformation,
Endovascular interventions:
- stationary balloon occlusion of feeding arteries - in-flow embolization (uncontrolled);
- combination of temporary or permanent balloon occlusion with in-flow embolization;
- superselective embolization.
Arteriovenous malformation is also treated with radiosurgery (Gamma-knife, Cyber-knife, Li nac, etc.).
- Embolization: Embolization is a procedure that inserts medical materials or glue into blood vessels to block blood flow to the AVM. Embolization can be used as a preparatory step before surgery or as a stand-alone treatment. It can help reduce bleeding and the size of the AVM.
- Radiation therapy: Radiation therapy may be used to treat AVMs, especially in cases where other treatments may be too risky. Radiation therapy aims to reduce the bleeding of the AVM and may require multiple sessions.
- Medication: In some cases, medications may be used to reduce symptoms such as pain or cramps. Medications may also be used to control blood pressure to reduce the risk of bleeding.
- Observation and symptom management: In some situations, especially if the AVM is small and not causing symptoms, a decision may be made to simply observe the condition and manage symptoms as needed.
Treatment for AVMs must be individualized for each patient, and the decision to choose a method depends on the specific circumstances. It is important to discuss all treatment options with a healthcare professional and conduct a thorough evaluation of the patient to determine the best treatment plan. Treatment results can be successful, and patients can achieve improvement or complete recovery, but each case is unique.
Forecast
The prognosis of an arteriovenous malformation (AVM) depends on several factors, including its size, location, symptoms, patient age, and success of treatment. It is important to remember that an AVM is a medical condition that can present differently in different patients, and the prognosis may vary from person to person. Here are some general aspects of the prognosis of an AVM:
- Risk of bleeding: The main risk of an AVM is the risk of bleeding (hemorrhage) in the brain. Small AVMs with a low risk of bleeding may have a good prognosis and not cause serious problems. However, large and medium-sized AVMs can pose a significant risk.
- Symptoms: Symptoms associated with an AVM, such as headaches, seizures, paralysis, or sensory disturbances, can affect the prognosis. In some cases, successful treatment can improve or eliminate symptoms.
- Size and location: AVMs located in more dangerous locations, such as deep in the brain or near critical structures, may have a worse prognosis. However, even many large AVMs can be successfully treated.
- Treatment: Treatment for AVMs may include surgical removal, embolization, radiation therapy, or medication. Successful treatment can reduce the risk of bleeding and improve the prognosis.
- Age: The patient's age can also affect the prognosis. Children and young adults often have a better prognosis than older patients.
- Comorbidities: The presence of other medical conditions or risk factors, such as high blood pressure or bleeding disorders, may affect the prognosis and treatment of AVM.
It is important to note that AVM requires careful medical monitoring and treatment. The decision on the method of treatment and prognosis should be made by qualified neurosurgeons and neuroradiologists based on an extensive evaluation of each individual case.

