Medical expert of the article
New publications
Human immunodeficiency virus (HIV)
Last reviewed: 04.07.2025

All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.
Acquired immunodeficiency syndrome was identified as a specific disease in 1981 in the United States, when a number of young people developed serious illnesses caused by microorganisms that were non-pathogenic or weakly pathogenic for healthy people. A study of the immune status of patients revealed a sharp decrease in the number of lymphocytes in general and T-helpers in particular. This condition was called AIDS (Acquired Immune Deficiency Syndrome). The method of infection (sexual contact, through blood and its preparations) indicated the infectious nature of the disease.
The causative agent of AIDS was discovered in 1983 independently by the Frenchman L. Montagnier, who called it LAV Lymphoadenopathy Associated Virus, since he found it in a patient with lymphadenopathy; and the American R. Gallo, who called the virus HTLV-III (Human T-lymphotropic Virus III): he had previously discovered lymphotropic viruses I and II.
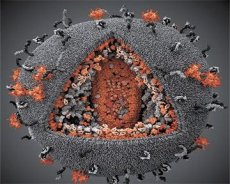
A comparison of the properties of the LAV and HTLV-III viruses showed their identity, so to avoid confusion, the virus was named HIV (Human Immunodeficiency Virus, or HIV) in 1986. HIV is spherical in shape, with a diameter of 110 nm. The virus envelope has the shape of a polyhedron, composed of 12 pentagons and 20 hexagons. In the center and corners of each hexagon is a molecule of glycosylated protein gpl20 (the number 120 indicates the molecular weight of the protein in kilodaltons). A total of 72 gpl20 molecules are located on the surface of the virion in the form of peculiar spikes, each of which is associated with the intramembrane protein gp41. These proteins, together with the double lipid layer, form the supercapsid (membrane) of the virion.
The gpl20 and gp41 proteins are formed by cutting the Env precursor protein by a cellular protease. The gp41 protein forms the spike "stem" by binding with its cytoplasmic domain to the matrix protein p17MA located directly under the envelope. The p17 molecules interact during virion maturation to form an icosahedron underlying the envelope.
In the central part of the virion, the p24 protein forms a cone-shaped capsid. The narrowed part of the capsid is connected to the virion membrane with the participation of the rb protein. Inside the capsid, there are two identical molecules of viral genomic RNA. They are connected by their 5' ends to the nucleocapsid protein p7NC. This protein is interesting because it has two amino acid residues (motifs) rich in cysteine and histidine and containing a Zn atom - they are called "zinc fingers" because they capture molecules of genomic RNA for inclusion in the forming virions. The capsid also contains three enzymes. Revertase (RT), or pol complex, includes reverse transcriptase, RNase H and DNA-dependent DNA polymerase. Revertase is present as a heterodimer p66/p51. Protease (PR) - p10, initiates and implements the process of virion maturation. Integrase (IN) - p31, or endonuclease, ensures the inclusion of proviral DNA into the host cell genome. The capsid also contains a molecule of primer RNA (tRNAl"3).
The RNA genome in the cell is converted into a DNA genome (DNA provirus) with the help of reverse transcriptase, consisting of 9283 nucleotide pairs. It is bounded on the left and right by so-called long terminal repeats, or LTRs: S'-LTR on the left and 3'-LTR on the right. LTRs contain 638 nucleotide pairs each.
The HIV genome consists of 9 genes, some of which overlap at the ends (have several reading frames) and have an exonintron structure. They control the synthesis of 9 structural and 6 regulatory proteins.
The importance of LTRs for the viral genome is that they contain the following regulatory elements that control its functioning:
- transcription signal (promoter region);
- poly-A addition signal;
- capping signal;
- integration signal;
- positive regulation signal (TAR for TAT protein);
- negative regulatory element (NRE for NEF protein);
- a site for attachment of primer RNA (tRNA™3) for minus-strand DNA synthesis at the 3' end; a signal at the 5' end of the LTR that serves as a primer for plus-strand DNA synthesis.
In addition, LTR contains elements involved in the regulation of mRNA splicing, packaging of vRNA molecules into the capsid (Psi element). Finally, during genome transcription, two signals are formed in long mRNAs for the REV protein, which switch protein synthesis: CAR - for regulatory proteins and CRS - for structural proteins. If the REV protein binds to CAR, structural proteins are synthesized; if it is absent, only regulatory proteins are synthesized.
The following regulatory genes and their proteins play a particularly important role in regulating the functioning of the virus genome:
- TAT protein, which carries out positive control of viral replication and acts through the TAR regulatory region;
- NEV and VPU proteins, which exert negative control of replication through the NRE region;
- REV protein, which carries out positive-negative control. REV protein controls the work of gag, pol, env genes and carries out negative regulation of splicing.
Thus, HIV replication is under triple control - positive, negative and positive-negative.
The VIF protein determines the infectivity of the newly synthesized virus. It is associated with the p24 capsid protein and is present in the virion in the amount of 60 molecules. The NEF protein is represented in the virion by a small number of molecules (5-10), possibly associated with the envelope.
The VPR protein inhibits the cell cycle at the G2 phase, participates in the transport of preintegration complexes into the cell nucleus, activates some viral and cellular genes, and increases the efficiency of virus replication in monocytes and macrophages. The location of the VPR, TAT, REV, and VPU proteins in the virion has not been established.
In addition to its own proteins, the virion membrane may contain some proteins of the host cell. VPU and VPR proteins participate in the regulation of viral reproduction.
Antigenic variants of human immunodeficiency virus (HIV)
The human immunodeficiency virus (HIV) is very variable. Even from the body of one patient, virus strains can be isolated that differ significantly in antigenic properties. Such variability is facilitated by the intensive destruction of CD4+ cells and a powerful antibody response to HIV infection. A new form of HIV, HIV-2, biologically close to HIV-1 but immunologically different from it, has been isolated from patients from West Africa. The homology of the primary structure of the genomes of these viruses is 42%. The DNA provirus HIV-2 contains 9671 bp, and its LTR - 854 bp. HIV-2 was subsequently isolated in other regions of the world. There is no cross-immunity between HIV-1 and HIV-2. Two large forms of HIV-1 are known: O (Outlier) and M (Major), the latter is divided into 10 subtypes (AJ). Eight subtypes (A-H) circulate in Russia.
 [ 1 ], [ 2 ], [ 3 ], [ 4 ], [ 5 ], [ 6 ], [ 7 ], [ 8 ], [ 9 ], [ 10 ]
[ 1 ], [ 2 ], [ 3 ], [ 4 ], [ 5 ], [ 6 ], [ 7 ], [ 8 ], [ 9 ], [ 10 ]
The mechanism of interaction of HIV with the cell
Having penetrated the body, the virus first attacks cells containing the CD4 receptor specific to it. This receptor is present in large quantities in T-helpers, in smaller quantities in macrophages and monocytes, and T-helpers are especially sensitive to the virus.
Human immunodeficiency virus (HIV) recognizes CD4 receptors using its gpl20 protein. The process of HIV interaction with the cell occurs according to the following scheme: receptor-mediated adsorption -> coated pit -> coated vesicle -> lysosome. In it, the virion membrane fuses with the lysosome membrane, and the nucleocapsid, freed from the supercapsid, enters the cytoplasm; on the way to the nucleus, it is destroyed, and genomic RNA and associated core components are released. Then, reverse transcriptase synthesizes the minus strand of DNA on the virion RNA, then RNase H destroys the virion RNA, and viral DNA polymerase synthesizes the plus strand of DNA. 5'-LTR and 3'-LTR are formed at the ends of the DNA provirus. The DNA provirus can remain in the nucleus for some time in an inactive form, but sooner or later it is integrated into the chromosome of the target cell with the help of its integrase. In it, the provirus remains inactive until the given T-lymphocyte is activated by microbial antigens or other immunocompetent cells. Activation of cellular DNA transcription is regulated by a special nuclear factor (NF-kB). It is a DNA-binding protein and is produced in large quantities during the activation and proliferation of T-lymphocytes and monocytes. This protein binds to certain sequences of cellular DNA and similar LTR sequences of the DNA provirus and induces transcription of both cellular DNA and DNA provirus. By inducing transcription of the DNA provirus, it carries out the transition of the virus from an inactive state to an active one and, accordingly, from a persistent infection to a productive one. The provirus can remain in an inactive state for a very long time. Activation of the virus is a critical moment in its interaction with the cell.
From the moment the virus penetrates the cell, the period of HIV infection begins - the virus carrier state, which can last 10 years or more; and from the moment the virus is activated, the disease begins - AIDS. With the help of its regulatory genes and their products, the virus begins to actively reproduce. TAT protein can increase the rate of virus reproduction by 1000 times. Viral transcription is complex. It includes the formation of both full-length and subgenomic mRNA, mRNA splicing, and then the synthesis of structural and regulatory proteins occurs.
Synthesis of structural proteins occurs as follows. First, the Pr55Gag precursor polyprotein (a protein with a molecular weight of 55 kDa) is synthesized. It contains 4 main domains: matrix (MA), capsid (CA), nucleocapsid (NC), and the rb domain, from which, as a result of cutting Pr55Gag by the viral protease (it is self-cut from another precursor protein, Gag-Pol), the structural proteins p17, p24, p7, and rb are formed, respectively. Formation of the Pr55Gag polyprotein is the main condition for the formation of viral particles. It is this protein that determines the virion morphogenesis program. It sequentially includes the stages of transport of the Gag polyprotein to the plasma membrane, interaction with it, and protein-protein interactions during the formation of the viral particle and its budding. Pr55Gag is synthesized on free polyribosomes; Protein molecules are transported to the membrane, where they are anchored by their hydrophobic regions. The CA domain plays the main role in creating the native conformation of the Gag protein. The NC domain ensures the inclusion (with the help of its "zinc fingers") of 2 molecules of genomic RNA in the composition of the forming viral particle. The polyprotein molecule initially dimerizes due to the interaction of the matrix domains. Then the dimers combine into hexameric (of 6 units) complexes as a result of the interaction of the CA and NC domains. Finally, the hexamers, joining at their lateral surfaces, form immature spherical virions, inside which the genomic viral RNA is contained, captured by the NC domain.
Another precursor protein, Prl60Gag-Pol (a protein with a molecular weight of 160 kDa), is synthesized as a result of a frameshift by the ribosome during translation of the 3'-end of the gag gene in the region located immediately upstream of the region encoding the rb protein. This Gag-Pol polyprotein contains an incomplete Gag protein sequence (1-423 amino acids) and Pol sequences that include the PR, RT, and IN domains. Gag-Pol polyprotein molecules are also synthesized on free polyribosomes and are transported to the plasma membrane. The Prl60Gagpol polyprotein contains all the intermolecular interaction sites and membrane binding sites inherent in the Gag polyprotein. Therefore, the Gag-Pol polyprotein molecules fuse with the membrane and, along with the Gag molecules, are included in the forming virions, resulting in the appearance of active protease and the beginning of the virion maturation process. HIV-1 protease is highly active only in the form of a dimer, therefore, for its self-excision from Prl60Gag-Pol, dimerization of these molecules is required. Virion maturation consists of the fact that the released active protease cuts prl60Gag-Pol and Gag55 in the sites recognized by it; proteins p17, p24, p7, p6, revertase, integrase are formed and their association into the viral structure occurs.
The Env protein is synthesized on ribosomes associated with the membranes of the endoplasmic reticulum, then it is glycosylated, cut by a cellular protease into gp120 and gp41, and transported to the cell surface. In this case, gp41 penetrates the membrane and binds to the matrix domains of the Gag protein molecule associated with the inner surface of the membrane. This connection is preserved in the mature virion.
Thus, the assembly of viral particles consists of the aggregation of precursor proteins and associated RNA molecules on the plasma membrane of the host cell, the formation of immature virions and their release by budding from the cell surface. During budding, the virion surrounds itself with a cell membrane into which the gp41 and gp120 molecules are embedded. During budding or, possibly, after the release of virions, their maturation occurs, which is carried out with the help of a viral protease and consists of proteolytic cutting of the precursor proteins Pr55Gag and Prl60Gag-Pol into proteins of the mature virus and their association into certain structural complexes. The leading role in the processes of viral morphogenesis is played by the precursor polyprotein Pr55Gag, which organizes and assembles the immature virion; the process of its maturation is completed by a specific viral protease.
Causes of immunodeficiency
One of the main causes of immunodeficiency in HIV infection is the mass death of T-helpers. It occurs as a result of the following events. First, T-helpers infected with the virus die due to apoptosis. It is believed that in AIDS patients, viral replication, apoptosis, and a decrease in the number of T-helpers are interconnected. Second, T-killers recognize and destroy T-cells infected with the virus or carrying adsorbed gpl20 molecules, as well as virus-infected and non-virus-infected T-helpers, which form symplasts (syncytium) consisting of several dozen cells (some of them die as a result of the reproduction of viruses in them). As a result of the destruction of a large number of T-helpers, there is a decrease in the expression of membrane receptors in B-lymphocytes to interleukin-2, the synthesis of various interleukins (growth factors and differentiation of B-lymphocytes - IL-4, IL-5, IL-6, etc.) is disrupted, resulting in a disruption of the function of the T-killer system. The activity of the complement and macrophage systems is suppressed. Macrophages and monocytes infected with the virus do not die for a long time, but they are not able to remove the virus from the body. Finally, due to the structural and antigenic similarity of gpl20 with the receptors of some epithelial cells of the body (including trophoblast receptors mediating the transplant transmission of HIV), antireceptor antibodies with a broad spectrum of action are synthesized. Such antibodies are able to block various cellular receptors and complicate the course of the disease with autoimmune disorders. The consequence of HIV infection is the defeat of all the main links of the immune system. Such patients become defenseless against a wide variety of microorganisms. This leads to the development of opportunistic infections and tumors. For patients with HIV infection, the risk of developing at least three types of cancer is increased: Kaposi's sarcoma; carcinoma (including skin cancer); B-cell lymphoma, which occurs due to the malignant transformation of B-lymphocytes. However, HIV is not only lymphocytotropic, but also neurotropic. It penetrates into the cells of the central nervous system (astrocytes) both by receptor-mediated endocytosis and by phagocytosis of virus-infected lymphoblasts by astrocytes. When the virus interacts with astrocytes, symplasts are also formed, which facilitate the spread of the pathogen through intercellular channels. The virus can persist in macrophages and monocytes for a long time, so they serve as a reservoir and distributors of it in the body, being able to penetrate all tissues. Infected macrophages play a major role in the introduction of HIV into the central nervous system and its damage. In 10% of patients, primary clinical syndromes are associated with damage to the central nervous system and manifest as dementia. Thus, people infected with HIV are characterized by 3 groups of diseases - opportunistic infections, tumor diseases and damage to the central nervous system.
Epidemiology of HIV infection
The source of HIV infection is only a person - a sick person or a virus carrier. The human immunodeficiency virus (HIV) is contained in blood, sperm, cervical fluid; in nursing mothers - in breast milk. Infection occurs sexually, through blood and its preparations, as well as from mother to child before, during and after childbirth. Cases of infection with the virus through food, drinks and insect bites are not known.
Drug addiction contributes to the spread of AIDS. The incidence of HIV is growing every year. According to the WHO, from 1980 to 2000, 58 million people were infected with HIV. In 2000 alone, 5.3 million people were infected worldwide, and 3 million people died from AIDS. As of January 1, 2004, 264 thousand HIV-infected people were registered in Russia. Half of people infected with HIV die within 11-12 years from the moment of infection. At the beginning of 2004, out of every 100 thousand citizens of Russia, about 180 lived with a diagnosis of "HIV infection". It is predicted that with this level of morbidity, the total number of HIV-infected people in Russia by 2012 will be 2.5-3 million people. The complexity of the fight against HIV infection depends on a number of reasons: firstly, there are no effective methods of its treatment and specific prevention; Secondly, the incubation period for HIV infection can exceed 10 years. Its duration depends on the moment of activation of the T-lymphocyte and the DNA provirus contained in its chromosome. It is still unclear whether everyone infected with the virus is doomed to AIDS or whether long-term carriage of the virus without disease is possible (which seems unlikely). Finally, there are several human immunodeficiency viruses (HIV-1, HIV-2), the antigenic differences between which prevent the formation of cross-immunity. The discovery of the simian immunodeficiency virus (SIV) shed light on the question of the origin of HIV. SIV is similar to HIV in its genome organization, but differs significantly in its nucleotide sequence. HIV-2 occupies an intermediate position between HIV-1 and SIV in its serological properties, and is closer to SIV in its nucleotide sequence. In this regard, V. M. Zhdanov suggested that HIV-1, HIV-2 and SIV viruses originated from a common ancestor. It is possible, according to R. Gallo, that one of the SIVs somehow entered the human body, where it underwent a series of mutations, resulting in the emergence of HIV-1, HIV-2 and its other forms.
Symptoms of HIV infection
The human immunodeficiency virus has some features that largely determine the pathogenesis of the disease. The virus has a very high reproduction rate, determined by its regulatory elements (up to 5,000 virions are synthesized in 5 minutes in the active stage). Due to the presence of the fusion protein (gp41), the virus induces the formation of extensive syncytial structures due to the fusion of infected and uninfected T-helpers, which results in their mass death. The gpl20 protein molecules formed in large quantities freely circulate in the blood and bind to the receptors of uninfected T-helpers, as a result of which they are also recognized and destroyed by T-killers. The virus can spread through intercellular channels from cell to cell, in which case it becomes poorly accessible to antibodies.
Clinical criteria for HIV infection
In adults, HIV infection is diagnosed when they have at least two serious symptoms in combination with at least one minor symptom and in the absence of other known causes of immunodeficiency (cancer, congenital immunodeficiency, severe starvation, etc.). Serious symptoms include:
- weight loss of 10% or more;
- a prolonged febrile condition, intermittent or constant;
- chronic diarrhea.
Minor symptoms: persistent cough, generalized dermatitis, recurrent herpes zoster, oral and pharyngeal candidiasis, chronic herpes simplex, generalized lymphadenopathy. The diagnosis of AIDS is made in the presence of only Kaposi's sarcoma, cryptococcal meningitis, pneumocystis pneumonia. The clinical picture of the disease is influenced by the accompanying opportunistic infection.
Methods of culturing human immunodeficiency virus (HIV)
HIV-1 and HIV-2 can be cultivated in cells of only one clone of TCV4 lymphocytes - H9, obtained from leukemic TCV4 lymphocytes. Monolayer cultures of astrocyte cells, in which HIV-1 reproduces well, can also be used for these purposes. Chimpanzees are susceptible to HIV-1 among animals.
The resistance of the virus in the external environment is low. It dies under the influence of sunlight and UV radiation, is destroyed at 80 °C within 30 minutes, when treated with commonly used disinfectants - within 20-30 minutes. To disinfect virus-containing material, it is necessary to use mycobactericidal disinfectants, since they are effective against microorganisms with the highest resistance.
Laboratory diagnostics of HIV infection
The main method for diagnosing virus carriage and HIV infection is the enzyme immunoassay. However, due to the fact that gpl20 has structural and antigen similarity to receptors of some human cells, including receptors that transport immunoglobulins through epithelial cells of the mucous membranes, antibodies related to antibodies against gpl20 may appear in the body. In this case, there may be false-positive ELISA results. Therefore, all positively reacting sera of the subjects undergo additional analysis using the immunoblotting method, or Western blotting. This method is based on the identification of the antibodies under study after their electrophoretic separation and subsequent testing using labeled anti-species antibodies. The virological method is rarely used due to the complexity of culturing the virus. The H9 lymphocyte clone is used to obtain viral antigens - the necessary components of diagnostic test systems. The CPR method allows detecting the virus at an early stage of viremia.
Treatment of HIV infection
It is necessary to find or synthesize drugs that effectively suppress the activity of reverse transcriptase (revertase) or viral protease. They would prevent the formation of DNA provirus and (or) inhibit intracellular reproduction of the virus. The modern strategy for treating HIV-infected patients is based on the principle of combined use of drugs that inhibit viral protease (one of the drugs) and reversease (2 different drugs) - combined (triple) therapy. In Russia, for the treatment of HIV-infected patients, the combined use of 2 domestic drugs is recommended: phosphazide and crixivan, which specifically suppress HIV reproduction at early and late stages of reproduction, especially with reduced activity of azidothymidine.
The problem of specific prophylaxis is the need to create a vaccine that would ensure the formation of effective cell-mediated immunity based on virus-specific cytotoxic lymphocytes without any significant production of antibodies. Such immunity is provided by Thl helpers. It is possible that antibodies, including virus-neutralizing ones, are not only ineffective in suppressing HIV infection, but at a high level they suppress cell-mediated immunity. Therefore, an anti-HIV vaccine must meet, first of all, two main requirements: a) be absolutely safe and b) stimulate the activity of T-cytotoxic lymphocytes. The effectiveness of various vaccine variants obtained from killed (inactivated) viruses and from individual antigens with high protective properties is being studied. Such antigens can either be isolated from the virions themselves or synthesized chemically. A vaccine created based on genetic engineering methods has been proposed. It is a recombinant vaccinia virus carrying HIV genes responsible for the synthesis of antigens with strong immunogenic properties. The question of the effectiveness of these vaccines requires considerable time due to the long incubation period of HIV infection and the high variability of the pathogen. The creation of a highly effective vaccine against HIV is an urgent fundamental problem.

