Medical expert of the article
New publications
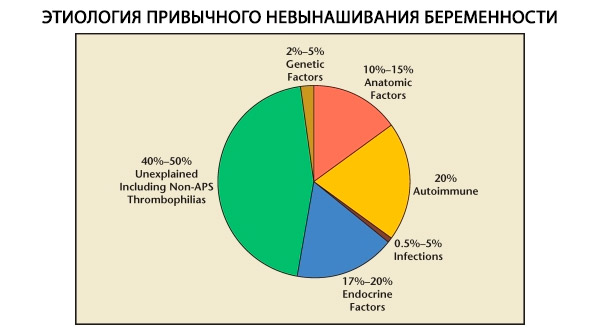
Habitual Unintended Pregnancy - Causes
Last reviewed: 04.07.2025

All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.
In the structure of habitual pregnancy losses, genetic, anatomical, endocrine, immunological and infectious factors are distinguished. When all the above causes are excluded, a group of patients remains in whom the origin of habitual miscarriage is unclear (idiopathic miscarriages). According to C. Coulam et al. (1996), 80% of idiopathic miscarriages are based on unrecognized immune disorders.
There is no convincing evidence that endometriosis causes recurrent miscarriage, or that medical or surgical treatment of endometriosis reduces the incidence of recurrent miscarriage.
According to current concepts, in addition to genetic and partially infectious causes leading to the formation of an abnormal embryo, the implementation of the damaging effect of other factors (anatomical, endocrine, immunological) consists in creating an unfavorable background for the development of a genetically complete fertilized egg, which leads to the depletion of the reserve capacity of the chorion and the cessation of development (embryogenesis). Critical periods in the first trimester of pregnancy are recognized as 6-8 weeks (death of the embryo) and 10-12 weeks (expulsion of the fertilized egg).
 [ 1 ]
[ 1 ]
Genetic causes of habitual miscarriage
Genetic factors account for 3–6% of the causes of habitual miscarriage. In sporadic termination of pregnancy in the first trimester, about 50% of abortions have chromosomal abnormalities. Most of them (95%) are changes in the number of chromosomes - monosomy (loss of one chromosome), trisomy (the presence of an additional chromosome), which are the result of errors in meiosis, as well as polyploidy (an increase in the chromosome composition by a full haploid set), which occurs when an egg is fertilized by two or more sperm. In sporadic miscarriages, trisomy is most often encountered - 60% of all mutations (most often on chromosome 16, as well as 13, 18, 21, 22), in second place in frequency is Shereshevsky-Turner syndrome (chromosome 45 X0) - 20%, the remaining 15% are accounted for by polyploidy (especially triploidy).
In the case of a change in the number of chromosomes in an abortion, the examination of the parents' karyotype most often does not reveal any pathology and the probability of a chromosomal disease of the fetus during a subsequent pregnancy is 1%. In contrast, when examining abortions in couples with habitual miscarriage, structural changes in chromosomes (intra- and interchromosomal) are observed in 3-6% of cases. When examining the parents' karyotype, balanced chromosomal rearrangements are found in 7% of cases. Most often, these are reciprocal translocations, in which a segment of one chromosome is located in the place of another segment of a non-homologous chromosome, as well as mosaicism of sex chromosomes, inversion and detection of chromosomes in the form of a ring. In the case of the presence of such rearrangements in one of the spouses, the processes of pairing and separation of chromosomes are hindered during meiosis, which results in the loss (deletion) or doubling (duplication) of chromosome sections in the gametes. This results in so-called unbalanced chromosomal rearrangements, in which the embryo is either nonviable or serves as a carrier of a severe chromosomal pathology. The probability of having a child with unbalanced chromosomal abnormalities in the presence of balanced chromosomal rearrangements in the karyotype of one of the parents is 1–15%. Differences in data are associated with the nature of the rearrangements, the size of the segments involved, the sex of the carrier, and family history.
Diagnostics
Anamnesis
- Hereditary diseases in family members.
- Presence of congenital anomalies in the family.
- Birth of children with mental retardation.
- The presence of infertility and/or miscarriage of unknown origin in a married couple or in relatives.
- Presence of unclear cases of perinatal mortality.
Special research methods
- A study of the parents' karyotype is especially indicated for married couples at the birth of a newborn with developmental defects in addition to a history of miscarriage, as well as in cases of habitual miscarriage in the early stages of pregnancy.
- Cytogenetic analysis of abortion in cases of stillbirth or neonatal mortality.
Indications for consultation with other specialists
If changes in the karyotype are detected in the parents, a consultation with a geneticist is necessary to assess the degree of risk of having a child with a pathology or, if necessary, to decide on the issue of donating an egg or sperm.
Further management of the patient
If a married couple has a pathological karyotype, even in one of the parents, it is recommended to conduct prenatal diagnostics during pregnancy - chorionic biopsy or amniocentesis - due to the high risk of developmental disorders in the fetus.
Anatomical causes of habitual miscarriage
Anatomical causes of habitual miscarriage include:
- congenital anomalies in the development of the uterus (complete doubling of the uterus; bicornuate, saddle-shaped, unicornuate uterus; partial or complete intrauterine septum);
- acquired anatomical defects;
- intrauterine adhesions (Asherman's syndrome);
- submucous uterine fibroids;
- isthmic-cervical insufficiency.
The frequency of anatomical anomalies in patients with habitual miscarriage ranges from 10 to 16%. The frequency of occurrence of uterine malformations that may result in miscarriage (but not infertility) in relation to all uterine malformations is as follows: bicornuate uterus - 37%, saddle-shaped uterus - 15%, intrauterine septum - 22%, complete doubling of the uterus - 11%, unicornuate uterus - 4.4%.
Diagnosis of habitual miscarriage
Anamnesis
In case of anatomical pathology of the uterus, late terminations of pregnancy and premature births are more often observed, however, with implantation on the intrauterine septum or near the myomatous node, early terminations of pregnancy are also possible.
For isthmic-cervical insufficiency, the pathognomonic sign is spontaneous termination of pregnancy in the second trimester or early premature birth, which occurs relatively quickly and with little pain.
In case of malformations of the uterus, it is necessary to pay attention to anamnestic indications of pathology of the urinary tract (often accompanying congenital anomalies of the uterus) and the nature of the development of menstrual function (indications of hematometra with a functioning rudimentary horn of the uterus).
Special examination methods
- Currently, hysterosalpingography is performed to establish a diagnosis, which allows studying the shape of the uterine cavity, identifying the presence of submucous fibroids, adhesions, septa, and determining the patency of the fallopian tubes. In order to diagnose uterine pathology, it is rational to perform hysterosalpingography in the period between menstruation and ovulation, i.e. in the first phase of the menstrual cycle after the cessation of bloody discharge (7-9th day of the cycle). To diagnose isthmic-cervical insufficiency, the study is performed in the second phase of the menstrual cycle (18-20th day) in order to determine the condition of the internal os of the cervix. Before performing hysterosalpingography, it is necessary to exclude inflammatory diseases of the pelvic organs or treat them.
- Hysteroscopy has become widespread in recent years and has become the gold standard for diagnosing intrauterine pathology. However, due to its higher cost compared to hysterosalpingography, the method is used in women with an indication of intrauterine pathology based on preliminary ultrasound data. Hysteroscopy can be used to examine the uterine cavity, determine the nature of the intrauterine pathology, and, if the necessary equipment (resectoscope) is available, perform minimally invasive surgical treatment - removal of adhesions, submucous myoma nodes, and endometrial polyps. When removing an intrauterine septum, preference is given to hysteroresectoscopy with laparoscopic control, which prevents the possibility of perforation of the uterine wall.
- Ultrasound is performed in the first phase of the menstrual cycle, which allows for a presumptive diagnosis of submucous uterine myoma, intrauterine adhesions, and in the second phase of the cycle - to identify an intrauterine septum and a bicornuate uterus. This method is of particular importance in the early stages of pregnancy, when its sensitivity in diagnosing these conditions is 100%, and its specificity is 80%. Outside of pregnancy, the diagnosis requires additional confirmation by other methods.
- Foreign authors point out the advantage of sonohysterography (ultrasound using a transvaginal sensor with preliminary introduction of 0.9% sodium chloride solution into the uterine cavity) over hysterosalpingography, as it allows differential diagnostics between the intrauterine septum and the bicornuate uterus. With sonohysterography, it is possible not only to study the shape of the uterine cavity, but also to determine the configuration of the fundus of the uterine body. In our country, this method has not become widespread.
- In some complex cases, MRI of the pelvic organs is used to verify the diagnosis. The method allows obtaining valuable information in case of uterine developmental anomalies accompanied by atypical arrangement of organs in the pelvis. MRI is important in case of a rudimentary uterine horn to decide whether it is advisable to remove it. The need to remove the rudimentary uterine horn occurs in case of its connection with the tube and ovary to prevent the formation and development of the fertilized egg in it. Termination of pregnancy in case of anatomical anomalies of the uterus may be associated with unsuccessful implantation of the fertilized egg (on the intrauterine septum, near the submucous myoma node), insufficiently developed vascularization and reception of the endometrium, close spatial relationships in the uterine cavity (for example, in case of cavity deformation by a myoma node), often accompanied by ICI, and hormonal disorders.
Treatment of habitual miscarriage
Surgical treatment
In the presence of an intrauterine septum, submucous myoma nodes and adhesions, the most effective surgical treatment is by hysteroresectoscopy. The frequency of subsequent miscarriages in this group of women after treatment is 10% compared to 90% before surgery. When comparing the results of metroplasty performed by laparotomy and transcervical hysteroresectoscopy, P. Heinonen (1997) obtained results indicating less trauma and greater effectiveness of hysteroresectoscopy; the percentage of pregnancies resulting in the birth of viable children was 68 and 86%, respectively.
Surgical removal of the intrauterine septum, adhesions, and submucous myoma nodes eliminates miscarriage in 70–80% of cases. However, it is ineffective in women with uterine malformations who have had normal births with subsequent recurrent miscarriages. It is likely that in such cases the anatomical factor is not the leading cause, and it is necessary to look for other causes of miscarriage.
It has been proven that abdominal metroplasty is associated with a significant risk of postoperative infertility and does not improve the prognosis of subsequent pregnancy. Therefore, it is better to give preference to hysteroscopy and laparoscopic operations.
Drug treatment
The effectiveness of the introduction of the IUD, high doses of estrogenic drugs, the introduction of a Foley catheter into the uterine cavity after operations to remove adhesions, intrauterine septum has not been proven. It is recommended to plan pregnancy no earlier than 3 months after the operation. To improve the growth of the endometrium, cyclic hormonal therapy is carried out for 3 menstrual cycles [14]. For 3 months in the first 14 days of the cycle, it is advisable to take a drug containing 2 mg of 17-beta-estradiol, in the next 14 days - 2 mg of 17-beta-estradiol and 20 mg of dydrogesterone (10 mg of dydrogesterone as part of a combination drug plus 10 mg of dydrogesterone in a separate tablet form).
Further management of the patient
Features of the course of pregnancy with a bicornuate uterus or doubling of the uterus (when there are 2 uterine cavities):
- in the early stages of pregnancy, bleeding often occurs from the "empty" horn or uterine cavity due to a pronounced decidual reaction; the tactics in this case should be conservative and consist of the use of antispasmodic and hemostatic agents;
- threat of termination of pregnancy at various stages;
- development of isthmic-cervical insufficiency;
- intrauterine growth retardation due to placental insufficiency.
In the early stages of pregnancy, in case of bleeding, bed and semi-bed rest are advisable, as well as the administration of hemostatic, antispasmodic and sedative drugs, and therapy with gestagens (dydrogesterone in a daily dose of 20 to 40 mg) up to 16–18 weeks of gestation.
Endocrine causes of habitual miscarriage
According to various authors, endocrine causes of miscarriage account for 8 to 20%. The most significant of these are luteal phase deficiency (LPD), hypersecretion of LH, thyroid dysfunction, and diabetes mellitus.
Severe thyroid disease or diabetes mellitus may lead to repeated miscarriages. However, in compensated diabetes mellitus, the risk of habitual miscarriages does not differ from that in the general population.
At the same time, the high incidence of hypothyroidism in the population requires screening with measurement of TSH levels. In patients with habitual miscarriage, luteal phase insufficiency is observed in 20–60% of cases, and ultrasound signs of polycystic ovaries - in 44–56%. According to the literature, the influence of individual hormonal disorders on the formation of the symptom complex of habitual miscarriage remains controversial. The studies of M. Ogasawara et al. (1997) did not reveal reliable differences in the frequency of termination of pregnancy with and without LPI in patients with two or more previous miscarriages in the anamnesis, excluding autoimmune, anatomical and infectious causes.
Insufficiency of the corpus luteum function can be the result of a number of unfavorable factors:
- disturbances in the secretion of FSH and LH in the first phase of the menstrual cycle;
- early or, conversely, too late peak of LH release;
- hypoestrogenism as a consequence of inadequate folliculogenesis. All these conditions are not subject to correction by replacement therapy with gestagen drugs in the postovulatory period. Prospective studies conducted by L. Regan et al. showed a significant increase in the frequency of miscarriages in patients with LH hypersecretion on the 8th day of the menstrual cycle compared to women with normal blood LH levels (65% and 12% of miscarriages, respectively). The damaging effect of an untimely surge of LH is associated with premature resumption of the second meiotic division and ovulation of an immature egg, as well as with the induction of androgen production by theca cells along with impaired endometrial reception under the influence of gestagen insufficiency. However, preliminary reduction of preovulatory LH levels with gonadotropin-releasing hormone agonists without additional measures aimed at prolonging subsequent pregnancy does not provide the expected reduction in the frequency of miscarriages.
The gold standard for diagnosing NLF is histological examination of material obtained from endometrial biopsy in the second phase of the cycle over 2 menstrual cycles.
Diagnosis of other causes of ovulatory dysfunction, such as hyperprolactinemia, hypothyroidism, functional excess of androgens (ovarian or adrenal), must be accompanied by the prescription of appropriate treatment.
Diagnostics
History and physical examination
- History. Factors to consider: late menarche, irregular menstrual cycle (oligomenorrhea, amenorrhea, sudden weight gain, weight loss, infertility, habitual early miscarriages).
- Examination: body type, height, body weight, hirsutism, severity of secondary sexual characteristics, presence of striae, examination of mammary glands for galactorrhea.
- Functional diagnostic tests: measurement of rectal temperature during 3 menstrual cycles.
Special research methods
- Hormonal study:
- in the 1st phase of the menstrual cycle (7–8th day) – determination of the content of FSH, LH, prolactin, TSH, testosterone, 17-hydroxyprogesterone (17-OP), DHEAS;
- in the 2nd phase of the menstrual cycle (21–22 days) – determination of progesterone content (normative indicators of progesterone levels are very variable, the method cannot be used without taking into account other factors).
- Ultrasound:
- in the 1st phase of the menstrual cycle (5–7th day) – diagnosis of endometrial pathology, polycystic ovaries;
- in the 2nd phase of the menstrual cycle (20–21 days) – measurement of the thickness of the endometrium (normal 10–11 mm, correlates with the content of progesterone).
- An endometrial biopsy to verify NLF is performed 2 days before the expected menstruation (on the 26th day with a 28-day cycle). This method is used in cases where the diagnosis is unclear. To study changes in the endometrium in the so-called "implantation window" period, a biopsy is performed on the 6th day after ovulation.
Treatment
When diagnosing NLF (according to rectal temperature charts, the duration of the 2nd phase is less than 11 days, a stepwise increase in temperature is observed, insufficient secretory transformation of the endometrium according to endometrial biopsy data, low levels of progesterone in the blood serum), it is necessary to identify the cause of such disorders.
If NLF is accompanied by hyperprolactinemia, MRI of the brain is performed. An alternative method is X-ray of the skull (sella turcica region).
The first stage in hyperprolactinemia is to exclude pituitary adenoma, which requires surgical treatment. In the absence of significant changes, hyperprolactinemia is considered functional, and bromocriptine treatment is prescribed to normalize prolactin levels. The initial dose of bromocriptine is 1.25 mg/day for 2 weeks, after monitoring prolactin levels, if the indicators do not normalize, the dose is increased to 2.5 mg/day. With a significant increase in prolactin levels, the initial dose is 2.5 mg/day. If pregnancy occurs, bromocriptine should be discontinued.
If hypothyroidism is detected, the nature of the thyroid pathology is determined together with an endocrinologist. In any case, daily sodium levothyroxine therapy is indicated, the dose is selected individually until the TSH level is normalized. If pregnancy occurs, sodium levothyroxine treatment should be continued. The question of the advisability of increasing the dose in the first trimester of pregnancy is decided together with an endocrinologist after receiving the results of a hormonal examination (TSH level, free thyroxine).
The correction of NLF is carried out in one of two ways. The first way is ovulation stimulation, the second way is replacement therapy with progesterone preparations.
The first treatment option is ovulation stimulation with clomiphene citrate. This treatment method is based on the fact that most luteal phase disorders are laid down in the follicular phase of the cycle. Constantly reduced progesterone levels in the 2nd phase are a consequence of impaired folliculogenesis in the 1st phase of the cycle. This disorder will be corrected with greater success by low doses of clomiphene citrate in the early follicular phase than by prescribing progesterone in the 2nd phase of the cycle.
In the 1st cycle, the dose of clomiphene citrate is 50 mg/day from the 5th to the 9th day of the menstrual cycle. The effectiveness is monitored using rectal temperature charts, progesterone level measurements in the 2nd phase of the cycle, or dynamic ultrasound. If there is no sufficient effect in the 2nd cycle of ovulation stimulation, the dose of clomiphene citrate should be increased to 100 mg/day from the 5th to the 9th day of the cycle. The maximum possible dose in the 3rd cycle of ovulation stimulation is 150 mg/day. Such an increase in the dose is possible only if the drug is well tolerated (no intense pain in the lower abdomen and lower back and no other signs of ovarian hyperstimulation).
The second treatment option: replacement therapy with progesterone preparations, which promote full secretory transformation of the endometrium, which gives the necessary effect in patients with habitual miscarriage with preserved ovulation. In addition, in recent years it has been established that the administration of progesterone preparations has not only a hormonal, but also an immunomodulatory effect, suppressing rejection reactions from immunocompetent cells in the endometrium. In particular, a similar effect has been described for dihydrogesterone at a dose of 20 mg / day. For the purpose of replacement therapy, dydrogesterone is used at a dose of 20 mg / day orally or micronized progesterone vaginally at a dose of 200 mg / day. Treatment is carried out on the 2nd day after ovulation (the day after the increase in rectal temperature) and continues for 10 days. If pregnancy occurs, treatment with progesterone preparations should be continued.
Modern research has not confirmed the effectiveness of human chorionic gonadotropin in the treatment of recurrent miscarriage.
In hyperandrogenism (ovarian or adrenal genesis) in patients with habitual miscarriage, drug treatment is indicated due to the effect of androgens on the completeness of ovulation and the state of the endometrium. In case of a violation of the biosynthesis of adrenal androgens, their virilizing effect on the female fetus is possible, therefore steroid therapy is carried out in the interests of the fetus.
Hyperandrogenism of ovarian genesis (polycystic ovaries)
History, physical examination and special examination results
- History: late menarche, menstrual cycle disorders such as oligomenorrhea (usually primary, less often secondary). Pregnancies are rare, usually spontaneously interrupted in the first trimester, with long periods of infertility between pregnancies.
- Examination: hirsutism, acne, stretch marks, high body mass index (optional).
- Rectal temperature charts: anovulatory cycles alternate with cycles with ovulation and NLF.
- Hormonal examination: high testosterone levels, FSH and LH levels may be elevated, LH/FSH ratio is greater than 3. Ultrasound: polycystic ovaries.
Treatment
Non-drug treatment
Weight loss - diet therapy, physical activity.
Drug treatment
- Orlistat at a dose of 120 mg with each main meal. The duration of the course is determined taking into account the effect and tolerability.
- Preliminary testosterone reduction with preparations containing cyproterone acetate (2 mg) and EE (35 mcg) for 3 menstrual cycles.
- Contraceptive discontinuation, hormonal support of the second phase of the cycle (gestagen therapy) - dydrogesterone at a dose of 20 mg/day from the 16th to the 25th day of the menstrual cycle. If there is no spontaneous ovulation, proceed to the next stage.
- Stimulation of ovulation with clomiphene citrate at an initial dose of 50 mg/day from the 5th to the 9th day of the menstrual cycle with simultaneous therapy with gestagens (dydrogesterone at a dose of 20 mg/day from the 16th to the 25th day of the cycle) and dexamethasone (0.5 mg).
- In the absence of pregnancy, the dose of clomiphene citrate is increased to 100–150 mg/day with the administration of gestagens in the second phase of the cycle and dexamethasone (0.5 mg). It has been established that, although dexamethasone only reduces the level of adrenal androgens, ovulation and conception occur significantly more often with treatment with clomiphene citrate and dexamethasone than with the use of clomiphene citrate alone [12].
- Three cycles of ovulation stimulation are performed, after which a break of three menstrual cycles with gestagenic support and a decision on surgical treatment using laparoscopic access (wedge resection of the ovaries, laser vaporization) is recommended.
Further management of the patient
Pregnancy management should be accompanied by gestagenic support up to 16 weeks of pregnancy (dydrogesterone at a dose of 20 mg/day or micronized progesterone at a dose of 200 mg/day), dexamethasone is prescribed only in the first trimester of pregnancy. Monitoring is mandatory for timely diagnosis of isthmic-cervical insufficiency and, if necessary, its surgical correction.
Adrenal hyperandrogenism (pubertal and postpubertal adrenogenital syndrome)
Adrenogenital syndrome (AGS) is a hereditary disease associated with a disruption in the synthesis of hormones of the adrenal cortex due to damage to genes responsible for the synthesis of a number of enzyme systems. The disease is inherited in an autosomal recessive manner with the transmission of mutant genes from both parents, who are healthy carriers.
In 90% of cases, adrenogenital syndrome is caused by mutations in the CYP21B gene, leading to a disruption in the synthesis of 21-hydroxylase.
History, physical examination and special examination results
- History: late menarche, slightly prolonged menstrual cycle, possible oligomenorrhea, spontaneous abortions in the first trimester, possible infertility.
- Examination: acne, hirsutism, android body type (broad shoulders, narrow pelvis), clitoral hypertrophy.
- Rectal temperature charts: anovulatory cycles alternate with cycles with ovulation and NLF.
- Hormonal test: high levels of 17-OP, DHEAS.
- Ultrasound: ovaries are unchanged.
A pathognomonic sign outside of pregnancy is an increase in the concentration of 17-OP in the blood plasma.
Currently, a test with ACTH is used to diagnose the latent, non-classical form of adrenal hyperandrogenism. Synacthen is used for this test - a synthetic polypeptide that has the properties of endogenous ACTH, i.e. it stimulates the initial phases of synthesis of steroid hormones from cholesterol in the adrenal glands.
Synacthen test (an ACTH analogue): 1 ml (0.5 mg) of synacthen is injected subcutaneously into the shoulder, the initial content of 17-OP and cortisol is first determined in the morning 9-hour blood plasma sample. A control blood sample is taken 9 hours after the injection to determine the level of 17-OP and cortisol. The determination index (D) is then calculated using the formula:
D = 0.052 × 17-OP + 0.005 × Cortisol/17-OP - 0.018 × Cortisol/17-OP
If the D coefficient is less than or equal to 0.069, this indicates the absence of adrenal hyperandrogenism. If the D coefficient is more than 0.069, it should be considered that hyperandrogenism is caused by a dysfunction of the adrenal glands.
Drug treatment
The mainstay of treatment for hyperandrogenism due to 21-hydroxylase deficiency is glucocorticoids, which are used to suppress excess androgen secretion.
Further management of the patient
Due to the virilizing effect of maternal androgens on the fetus, with an established diagnosis of adrenal hyperandrogenism, treatment with dexamethasone at an initial dose of 0.25 mg is prescribed before pregnancy and continued at an individually selected dose (from 0.5 to 1 mg) throughout pregnancy. In a woman with habitual miscarriage suffering from adrenal hyperandrogenism, it is inappropriate to cancel treatment, since the frequency of miscarriages in the absence of treatment reaches 14%, with continuation - 9%.
Considering the fact that patients with adrenogenital syndrome can pass this gene to the fetus, it is necessary to conduct prenatal diagnostics: at 17-18 weeks of pregnancy, a blood test is prescribed to determine the content of 17-OP in the mother. If the level of the hormone in the blood is increased, its concentration in the amniotic fluid is determined. If the content of 17-OP in the amniotic fluid is increased, adrenogenital syndrome in the fetus is diagnosed. Unfortunately, it is impossible to determine the severity of adrenogenital syndrome (mild or salt-wasting severe form) by the level of 17-OP in the amniotic fluid. The question of maintaining the pregnancy in this situation is decided by the parents.
If the child's father is a carrier of the adrenogenital syndrome gene and there have been cases of children born with this syndrome in the family, then the patient, even without adrenal hyperandrogenism, receives dexamethasone in the interests of the fetus (to prevent virilization of the female fetus) at a dose of 20 mcg/kg of body weight, maximum 1.5 mg/day in 2-3 doses after meals. At 17-18 weeks, after deciding on the sex of the fetus and the expression of the adrenogenital syndrome gene (based on the results of amniocentesis), treatment is continued until the end of pregnancy if the fetus is a girl with adrenogenital syndrome. If the fetus is a boy or a girl who is not a carrier of the adrenogenital syndrome gene, dexamethasone can be discontinued.
If a woman with habitual miscarriage suffers from adrenal hyperandrogenism, then dexamethasone treatment is carried out throughout the pregnancy and is discontinued only after delivery. On the 3rd day after delivery, the dose of dexamethasone is gradually reduced (by 0.125 mg every 3 days) until complete discontinuation in the postpartum period.
Hyperandrogenism of mixed genesis (ovarian and adrenal)
History, physical examination and special examination results
- History: late menarche, menstrual cycle disorders such as oligomenorrhea (usually primary, less often secondary), amenorrhea, possible injuries, concussions. Pregnancies are rare, usually spontaneously interrupted in the first trimester, long periods of infertility between pregnancies.
- Physical examination: hirsutism, acne, striae, acanthosis nigricans, high body mass index, hypertension.
- Rectal temperature charts: anovulatory cycles alternate with cycles with ovulation and NLF.
- Hormonal examination: high testosterone levels, FSH and LH levels may be elevated, LH/FSH ratio greater than 3, high DHEAS, 17-OP levels, hyperprolactinemia may be present.
- Ultrasound: polycystic ovaries.
- Electroencephalography: changes in the bioelectrical activity of the brain.
- Hyperinsulinemia, lipid metabolism disorder (high cholesterol, low-density lipoproteins and very low-density lipoproteins), decreased glucose tolerance or elevated blood glucose levels.
Treatment
Non-drug treatment
Weight loss (low-calorie diet, physical activity).
Drug treatment
The first stage - in the presence of insulin resistance, it is recommended to prescribe metformin at a daily dose of 1000-1500 mg to increase insulin sensitivity.
The second stage - in case of severe menstrual cycle disorders and high testosterone levels, it is recommended to prescribe drugs with an antiandrogenic effect containing cyproterone acetate (2 mg) and ethinyl estradiol (35 mcg) for 3 months.
The third stage is stimulation of ovulation followed by gestagenic support (the scheme is described above) and taking dexamethasone at a daily dose of 0.25–0.5 mg.
In case of hyperprolactinemia and hypothyroidism, appropriate drug correction should be performed in ovulation stimulation cycles. If pregnancy occurs, bromocriptine should be discontinued and levothyroxine should be continued.
If ovulation stimulation is ineffective, the question of prescribing direct ovulation inducers, the advisability of surgical treatment of polycystic ovaries or in vitro fertilization should be decided.
Further management of the patient
In patients with metabolic syndrome, pregnancy is often complicated by arterial hypertension, nephropathy, hypercoagulation, in connection with which it is necessary to monitor blood pressure, hemostasiograms from the early stages of pregnancy and correct the arising disorders (if necessary) with antihypertensive drugs, antiplatelet agents and anticoagulants. Gestagenic drugs are prescribed up to 16 weeks of pregnancy - didrogesterone at a dose of 20 mg / day or micronized progesterone at a dose of 200 mg / day in 2 doses.
All women with hyperandrogenism represent a risk group for the development of isthmic-cervical insufficiency. Monitoring of the cervix condition should be carried out from the 16th week of pregnancy, if necessary - surgical correction of isthmic-cervical insufficiency.
Immunological causes of habitual miscarriage
It is currently known that about 80% of all previously unexplained cases of repeated pregnancy loss (after excluding genetic, anatomical, hormonal causes) are associated with immune disorders. Autoimmune and alloimmune disorders are distinguished, leading to habitual miscarriage.
In autoimmune processes, the immune system's own tissues become the object of aggression, i.e. the immune response is directed against its own antigens. In this situation, the fetus suffers secondarily as a result of damage to the mother's tissues.
In alloimmune disorders, a woman's immune response is directed against embryonic/fetal antigens received from the father and which are potentially foreign to the mother's body.
Autoimmune disorders most frequently encountered in patients with habitual miscarriage include the presence of antiphospholipid, antithyroid, and antinuclear autoantibodies in the serum. Thus, it has been established that 31% of women with habitual miscarriage outside pregnancy have autoantibodies to thyroglobulin and thyroid peroxidase (thyroid microsomal [thyroid peroxidase] autoantibodies); in these cases, the risk of spontaneous miscarriage in the first trimester of pregnancy increases to 20%. In habitual miscarriage, the presence of antinuclear and antithyroid antibodies indicates the need for further examination to identify the autoimmune process and verify the diagnosis.
Antiphospholipid syndrome (APS) remains a generally recognized autoimmune condition leading to embryo/fetal death.
Alloimmune disorders
Currently, alloimmune processes leading to fetal rejection include the presence of an increased (more than 3) number of common antigens of the major histocompatibility complex system in spouses (often observed in consanguineous marriages); low levels of blocking factors in the mother's serum; increased levels of natural killer cells (NK cells CD56, CD16) in the endometrium and peripheral blood of the mother both outside and during pregnancy; high levels of concentration of a number of cytokines in the endometrium and blood serum, in particular, γ-interferon, tumor necrosis factor a, interleukins-1 and 2.
Currently, alloimmune factors leading to early pregnancy losses and ways to correct the above conditions are under study. There is no consensus on the methods of therapy. According to some researchers, active immunization with donor lymphocytes does not produce a significant effect, while other authors describe a significant positive effect with such immunization and treatment with immunoglobulins.
Currently, one of the immunomodulatory agents in early pregnancy is progesterone. In particular, studies have proven the role of dydrogesterone in a daily dose of 20 mg in women with habitual miscarriage in the first trimester of pregnancy with an increased level of CD56 cells in the endometrium.
 [ 11 ], [ 12 ], [ 13 ], [ 14 ], [ 15 ], [ 16 ]
[ 11 ], [ 12 ], [ 13 ], [ 14 ], [ 15 ], [ 16 ]
Genetically determined thrombophilias
The following forms of genetically determined thrombophilias are considered to be thrombophilic conditions during pregnancy leading to habitual miscarriage.
- Antithrombin III deficiency.
- Factor V mutation (Leiden mutation).
- Protein C deficiency.
- Protein S deficiency.
- Prothrombin gene mutation G20210A.
- Hyperhomocysteinemia.
Examination to identify rare causes of thrombophilia is necessary in cases where there were:
- family history - thromboembolism before the age of 40 in relatives;
- reliable episodes of venous and/or arterial thrombosis before the age of 40 years;
- recurrent thrombosis in the patient and immediate relatives;
- thromboembolic complications during pregnancy and after childbirth when using hormonal contraception;
- repeated pregnancy losses, stillbirths, intrauterine growth retardation, placental abruption;
- early onset preeclampsia, HELLP syndrome.
Infectious causes of habitual miscarriage
The role of the infectious factor as a cause of habitual miscarriage is currently widely debated. It is known that primary infection in the early stages of pregnancy may cause damage to the embryo that is incompatible with life, which leads to sporadic spontaneous miscarriage. However, the probability of reactivation of the infection at the same time with the outcome of repeated pregnancy losses is negligible. In addition, microorganisms that provoke habitual miscarriage have not been found at present. Studies in recent years have shown that most women with habitual miscarriage and chronic endometritis have a prevalence of 2-3 or more types of obligate anaerobic microorganisms and viruses in the endometrium.
According to V.M. Sidelnikova et al., in women suffering from habitual miscarriage, the diagnosis of chronic endometritis outside pregnancy was histologically verified in 73.1% of cases and in 86.7%, persistence of opportunistic microorganisms in the endometrium was observed, which can certainly be the cause of activation of immunopathological processes. Mixed persistent viral infection (herpes simplex virus, Coxsackie A, Coxsackie B, enteroviruses 68–71, cytomegalovirus) is found in patients with habitual miscarriage significantly more often than in women with a normal obstetric history. K. Kohut et al. (1997) showed that the percentage of inflammatory changes in the endometrium and decidual tissue in patients with primary recurrent miscarriage is significantly higher than in women after miscarriage with a history of at least one full-term birth.
Bacterial and viral colonization of the endometrium usually results from the inability of the immune system and non-specific protective forces of the body (the complement system, phagocytosis) to completely eliminate the infectious agent, and at the same time, its spread is limited by the activation of T-lymphocytes (T-helpers, natural killers) and macrophages. In all the above cases, persistence of microorganisms occurs, characterized by the attraction of mononuclear phagocytes, natural killers, T-helpers, synthesizing various cytokines, to the site of chronic inflammation. Apparently, such a state of the endometrium prevents the creation of local immunosuppression in the preimplantation period, which is necessary to form a protective barrier and prevent rejection of a half-foreign fetus.
In this regard, before pregnancy, women with habitual miscarriage should be diagnosed with chronic endometritis. To establish or exclude this diagnosis, an endometrial biopsy is performed on the 7th-8th day of the menstrual cycle with histological examination, PCR and bacteriological examination of the material from the uterine cavity. When verifying the diagnosis, chronic endometritis is treated according to the standards for the treatment of inflammatory diseases of the pelvic organs.

