Medical expert of the article
New publications
Fusobacteria: friend or foe?
Last reviewed: 06.07.2025

All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.
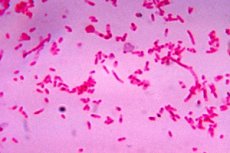
According to the classification accepted in microbiology, fusobacteria are prokaryotes and are gram-negative anaerobic bacteria that live in the body of humans and other mammals, being part of the constant normal microbiocenosis or microflora. Their family - Fusobacteriaceae - has more than one and a half dozen species.
Morphology of Fusobacteria and Features of Their Metabolism
Fusobacteria have a single-celled, spindle-shaped structure (fusus in Latin) due to the pointed ends on both sides. The rods can be thick and thin, straight and curved, and can also be filiform. The length of these bacteria ranges from 0.0005 to 0.008 mm, and they have no organs of movement, although some sources claim that they have peritrichous (located over the entire surface) flagella.
Bacteriologists note that these microorganisms do not form spores, that is, in the event of deterioration of living conditions, they cannot turn into cells with a dense membrane. Fusobacteria reproduce by mitotic splitting of one cell into two with horizontal transfer of genes concentrated in the nucleoid.
The morphology of fusobacteria partly determines the habitats of their colonies: the mucous membranes of the oral cavity, respiratory tract, urogenital area and the lower part of the digestive tract - the large intestine. Their presence in the blood has not been established, but fusobacteria do not need this, because they receive nutrients through oil fermentation of glucose, sucrose, maltose and some amino acids.
So the basis of the metabolism of these microorganisms is the biochemical process of anaerobic (without oxygen) dissimilation of carbohydrates under the influence of enzymes. The metabolites are low-molecular butyric (butanoic) acid, carbon dioxide and hydrogen. To obtain energy, bacteria need hydrogen, and its ions are accepted by the surface protein of fusobacteria adhesin A (FadA), and then moved into the cell.
By the way, butyric acid is very important for maintaining intestinal homeostasis (absorption of water and electrolytes) and for the regeneration of mucous epithelial cells; doctors have established a relationship between the deficiency of this acid in the intestine and the development of local inflammatory pathologies (for example, ulcerative colitis). In addition to fusobacteria, butyric acid is produced by bacteria of the genus Clostridium.
On the conditional pathogenicity of fusobacteria
Fusobacteria, like most gram-negative anaerobes, are considered by bacteriologists to be opportunistic pathogens, but there are strains whose increased pathogenicity scientists no longer doubt. In particular, this includes Fusobacterium necrophorum, which lives in the oral cavity and intestines, and Fusobacterium nucleatum, which has chosen dental plaque for its habitat.
How does their pathogenic mechanism work? The outer surface of the cytoplasmic membrane of fusobacteria consists of polymerized fats, proteins and carbohydrates in the form of lipopolysaccharides, which are bacterial toxic substances (endotoxins) and, at the same time, antigens. That is, these compounds cause an immune response of the body and an inflammatory reaction without obvious exogenous (external) impact on individual systems and organs.
There is an opinion that the pathogenicity of some bacteria of the Fusobacteriaceae family manifests itself only in the case of weakened immunity, however, it should be taken into account that they have been shown to have the ability to be highly aggressive, since fusobacteria produce phospholipase A - an enzyme that breaks down lipids of cell membranes and opens access for bacteria to cells of all tissues. But microorganisms, as a rule, do not use this enzyme "alone", but in the presence of pathogenic microorganisms, the activity increases significantly. When the mucous membrane is damaged by streptococcus or staphylococcus, fusobacteria, taking advantage of the opportunity, penetrate deeper and cause necrotic inflammation of tissues. The most illustrative example of such a synergistic effect is gangrenous pharyngitis (or Simanovsky-Plaut-Vincent's angina), which occurs due to infection of the mucous membrane by gram-negative bacteria Spirochaetales Borrelia vincentii, Prevotella intermedia and Fusobacterium nucleatum.
What diseases are caused by fusobacteria?
Now let's list some diseases caused by fusobacteria, or more precisely, pathologies that develop with their more than active participation. Doctors include the following among them:
- pulpitis of carious teeth;
- gingivitis;
- periodontal disease (periodontitis);
- osteomyelitis of the jaw;
- phlegmons of various localizations;
- tonsillitis and paratonsillitis (phlegmonous tonsillitis);
- chronic sinusitis;
- retropharyngeal abscess after streptococcal tonsillitis with necrosis and sepsis (Lemierre's syndrome);
- bronchiectasis;
- purulent pneumonia;
- lung abscess;
- empyema of the pleura;
- brain abscesses;
- purulent inflammation of the abdominal organs;
- erosive balanitis and balanoposthitis;
- acute colpitis (vaginitis) and vulvitis;
- purulent-septic complications of medical abortions;
- ulcerative colitis;
- Crohn's disease;
- septicemia.
Researchers from Harvard Medical School and the Dana-Faber Cancer Institute conducted a genomic analysis of colorectal cancer tumors and found an abnormally large number of fusobacteria F. nucleatum in them. To date, studies are ongoing to confirm (or refute) the hypothesis that fusobacteria are involved in the development of colon and rectal cancer. The fact is that the bacterial surface protein adhesin A (which we mentioned above) binds to the transmembrane glycoprotein of human epithelial cells E-cadherin. This protein ensures intercellular adhesion in our tissues and can “glue” cancer cells together, preventing their invasion. But fusobacteria neutralize it, as a result of which unhindered proliferation of cancer cells begins. [ 4 ], [ 5 ]
Treatment of Fusobacteria
Treatment of fusobacteria, or rather, drug therapy for fusobacterial diseases, is carried out with antibiotics.
Among antibacterial drugs, doctors prefer those that are most active against F. nucleatum and F. Necrophorum: clindamycin, carbenicillin, cefoxitin, cefoperazone, cefamandole, fosfimycin, ornidazole. The prescription of a specific drug, naturally, depends on the diagnosis and clinical picture of the disease.
Carbenicillin (trade names: Carbecin, Fugacillin, Microcillin, Pyocyanil, etc.) acts only on gram-negative bacteria and is used in cases of peritonitis, septicemia, meningitis, osteomyelitis as part of complex therapy.
The second-generation cephalosporin antibiotic Cefoxitin (Mefoxin, Atralxitin, Boncefin) is recommended for a wide range of diseases of bacterial etiology, including tonsillitis, pneumonia, urinary tract infections, bones, joints, skin, soft tissues; it is prescribed to prevent infectious complications after surgery.
And the drug Fosfomycin (Fosfomycin trometamol, Monural, Urofosfabol) is used for urological bacterioses - recurrent cystitis or non-specific urethritis (single dose 3 g).
At the beginning of the article, we promised to find out the degree of danger to humans of the tiny single-celled fusobacterium. Yes, it can be pathogenic, but, on the other hand, a person cannot get rid of its presence in the microflora.


 [
[