Medical expert of the article
New publications
Causes and pathogenesis of diphtheria
Last reviewed: 04.07.2025

All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.
Diphtheria is a paradigm of toxigenic infectious diseases. In 1883, Klebs demonstrated that Corynebacterium diphtheriae was the causative agent of diphtheria. A year later, Loeffler found that the organism could be cultured only from the nasopharyngeal cavity and proposed that the damage to internal organs was due to a soluble toxin. By 1888, Roux and Yersin showed that animals injected with sterile filtrates of C. diphtheriae developed organ pathology indistinguishable from that of human diphtheria; this demonstrated that a potent exotoxin was the major virulence factor.
Diphtheria is most often an upper respiratory tract infection causing fever, sore throat, and malaise. A thick, gray-green fibrin membrane, a pseudomembrane, often forms at the site(s) of infection as a result of the combined effects of bacterial growth, toxin production, underlying tissue necrosis, and the host immune response. Recognition that systemic organ damage is due to the action of diphtheria toxin has led to the development of both effective antitoxin-based therapy for the treatment of acute infection and a highly effective toxoid vaccine.
Although toxoid immunization has made diphtheria a rare disease in areas where public health standards require vaccination, outbreaks of diphtheria still occur in unimmunized and immunocompromised groups. In contrast, widespread outbreaks of diphtheria reaching epidemic proportions have occurred in areas where active immunization programs have been suspended.
Other types of corynebacteria
In addition to C. diphtheriae, C. ulcerans, and C. pseudotuberculosis, C. pseudodiphtheriticum and C. xerosis may occasionally cause nasopharyngeal and skin infections. The latter two strains are recognizable by their ability to produce pyrazinamidase. In veterinary medicine, C. renale and C. kutscheri are important pathogens, causing pyelonephritis in cattle and latent infections in mice, respectively.
Cause
The causative agent of diphtheria, Corynebacterium diphtheriae, is a thin, slightly curved rod with club-shaped thickenings at the ends, motionless; it does not form spores, capsules or flagella, and is gram-positive.
In addition to the toxin, diphtheria corynebacteria produce neuraminidase, hyaluronidase, hemolysin, necrotizing and diffuse factors during their life processes, which can cause necrosis and liquefaction of the main substance of connective tissue.
Based on their ability to form toxin, diphtheria corynebacteria are divided into toxigenic and non-toxigenic.
Diphtheria toxin is a potent bacterial exotoxin that determines both general and local clinical manifestations of the disease. Toxigenicity is genetically determined. Non-toxigenic corynebacteria of diphtheria do not cause the disease.
According to cultural and morphological features, all diphtheria corynebacteria are divided into 3 variants: gravis, mitis, intermedius. There is no direct dependence of the severity of the disease on the variant of diphtheria corynebacteria. Each variant contains both toxigenic and non-toxigenic strains. Toxigenic corynebacteria of all variants produce an identical toxin. [ 1 ]
Structure, classification and antigen types
Corynebacterium diphtheriae is a Gram-positive, non-motile, club-shaped rod. Strains growing in tissue or older in vitro cultures contain fine spots in the cell walls that allow decolorization during Gram staining and result in a variable Gram reaction. Older cultures often contain metachromatic granules (polymetaphosphate) that stain bluish-purple with methylene blue. Cell wall sugars include arabinose, galactose, and mannose. In addition, the toxic 6,6'-ester of trehalose may be isolated, containing corynemycolic and corynemycolenic acids in equimolar concentrations. Three distinct culture types are recognized: mitis, intermedius, gravis.
Most strains require nicotinic and pantothenic acids for growth; some also require thiamine, biotin, or pimelic acid. For optimal diphtheria toxin production, the medium must be supplemented with amino acids and must be set aside.
As early as 1887, Loeffler described the isolation of avirulent (nontoxigenic) C. diphtheriae that were indistinguishable from virulent (toxigenic) strains isolated from patients in healthy individuals. It is now recognized that avirulent strains of C. diphtheriae can be converted to a virulent phenotype following infection and lysogenization by one of a number of distinct corynebacteriophages carrying the structural gene for diphtheria toxin, tox. Lysogenic conversion of the avirulent to virulent phenotype can occur both in situ and in vitro. The structural gene for diphtheria toxin is not essential for either corynebacteriophage or C. diphtheriae. Despite this observation, genetic drift of diphtheria toxin has not been observed.
Pathogenesis
The entry points of infection are the mucous membranes of the oropharynx, nose, larynx, less often the mucous membrane of the eyes and genitals, as well as damaged skin, wound or burn surfaces, diaper rash, unhealed umbilical wound. At the entry point, the diphtheria corynebacterium multiplies and secretes exotoxin.
Exudate rich in fibrinogen is exuded and converted into fibrin under the influence of thrombokinase released during necrosis of epithelial cells. A fibrinous film is formed - a characteristic sign of diphtheria.
Asymptomatic nasopharyngeal carriage is common in diphtheria endemic regions. In susceptible individuals, toxigenic strains cause disease by replicating and secreting diphtheria toxin in the nasopharynx or skin lesions. The diphtheria lesion is often covered by a pseudomembrane composed of fibrin, bacteria, and inflammatory cells. Diphtheria toxin can be proteolytically cleaved into two fragments: N-terminal fragment A (catalytic domain) and fragment B (transmembrane and receptor-binding domains). Fragment A catalyzes NAD+-dependent ADP-ribosylation of elongation factor 2, thereby inhibiting protein synthesis in eukaryotic cells. Fragment B binds to a cell surface receptor and facilitates delivery of fragment A to the cytosol.
Protective immunity involves the antibody response to diphtheria toxin after clinical disease or to diphtheria toxin (toxin inactivated by formaldehyde) after immunization.
Colonization
Little is known about the factors that mediate colonization of C. diphtheriae. However, it is clear that factors other than diphtheria toxin production contribute to virulence. Epidemiological studies have shown that a given lysotype can persist in a population for long periods of time. It may later be replaced by another lysotype. The emergence and subsequent dominance of a new lysotype in a population is presumably related to its ability to colonize and compete effectively in its segment of the nasopharyngeal ecological niche. Corynebacterium diphtheriae can produce neuraminidase, which cleaves cell surface sialic acid into its components pyruvate and N-acetylneuraminic acid. Cord factor (6,6'-di-O-mycoloyl-α, α'-D-trehalose) is a surface component of C. diphtheriae, but its role in colonization of the human host is unclear.
Production of diphtheria toxins
The structural gene of diphtheria toxin, tox, belongs to a family of closely related corynebacteriophages, of which the β-phage is the best studied. Regulation of diphtheria toxin expression is mediated by an iron-activated repressor, DtxR, which is encoded by the C. diphtheriae genome. Toxin expression depends on the physiological state of C. diphtheriae. Under conditions in which iron becomes the growth-rate limiting substrate, iron dissociates from DtxR, the toxicodendron gene becomes activated, and diphtheria toxin is synthesized and secreted into the culture medium at maximal rates.
Diphtheria toxin is unusually potent; for susceptible species (e.g. humans, monkeys, rabbits, guinea pigs) as little as 100 to 150 ng/kg body weight is lethal. Diphtheria toxin consists of a single polypeptide chain of 535 amino acids. Biochemical, genetic, and X-ray structural analysis show that the toxin consists of three structural/functional domains:
- N-terminal ADP-ribosyltransferase (catalytic domain);
- a region that facilitates delivery of the catalytic domain across the cell membrane (transmembrane domain);
- eukaryotic cell receptor binding domain.
Following mild trypsin digestion and reduction under denaturing conditions, diphtheria toxin can be specifically cleaved at its protease-sensitive loop into two polypeptide fragments (A and B). Fragment A is the N-terminal 21 kDa component of the toxin and contains the catalytic site for ADP-ribosylation of elongation factor 2 (EF-2)
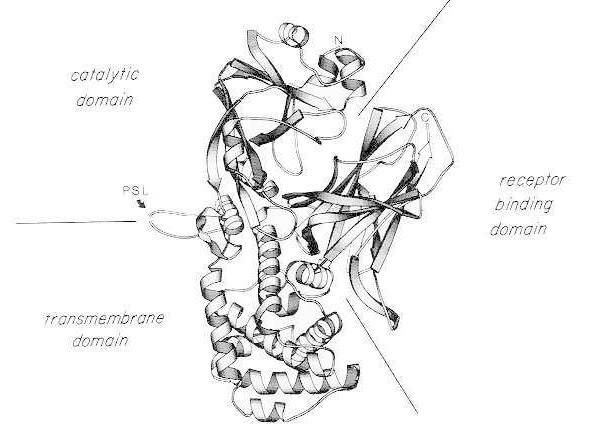
Ribbon diagram of the X-ray crystal structure of monomeric native diphtheria toxin. (modified from Bennett MJ, Choe S, Eisenberg D: Domain swapping: Entangling aliances between proteins. Proc Natl Acad Sci, USA, 91: 3127, 1994). The relative positions of the catalytic, transmembrane, and receptor-binding domains are shown. The intact toxin can be cleaved by trypsin-like proteases at Arg190, Arg192, and/or Arg193, which are located in the protease-sensitive loop (PSL). Following reduction of the disulfide bridge between Cys186 and Cys201, the toxin can be resolved into A and B fragments. The amino terminus (N) and carboxy terminus (C) of the intact toxin are shown. The strip chart was created using the MOLESCRIPT program.
The C-terminal fragment, fragment B, carries the transmembrane and receptor-binding domains of the toxin.
Poisoning of a single eukaryotic cell by diphtheria toxin involves at least four distinct steps:
- binding of the toxin to its receptor on the cell surface;
- clustering of charged receptors into coated pits and internalization of the toxin via receptor-mediated endocytosis; following acidification of the endocytic vesicle by a membrane-associated, ATP-driven proton pump,
- insertion of the transmembrane domain into the membrane and facilitated delivery of the catalytic domain into the cytosol, and
- ADP-ribosylation of EF-2, resulting in irreversible suppression of protein synthesis.
It has been shown that a single molecule of the catalytic domain delivered into the cytosol is sufficient to be lethal to the cell.
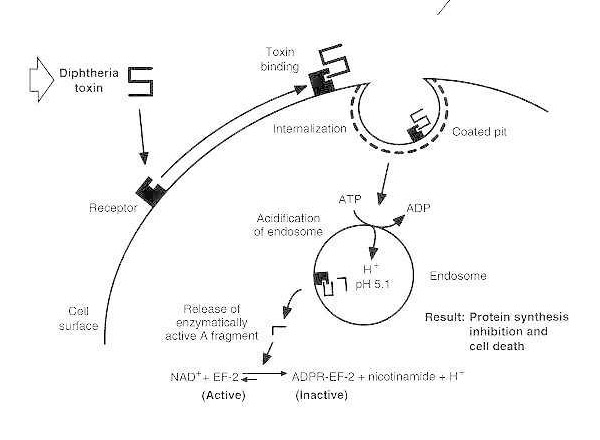
Schematic diagram of diphtheria intoxication of a susceptible eukaryotic cell.
The toxin binds to its cell surface receptor and is internalized via receptor-mediated endocytosis; upon acidification of the endosome, the transmembrane domain is inserted into the vesicle membrane; the catalytic domain is delivered to the cytosol, leading to inhibition of protein synthesis and cell death.
Epidemiology
Before mass immunization of the U.S. population with diphtheria toxoid, diphtheria was usually a childhood disease. A remarkable aspect of mass immunization with diphtheria toxoid is that as the percentage of the population with protective levels of antitoxin immunity (≥ 0.01 IU/mL) increases, the frequency of isolation of toxigenic strains from the population decreases. Today, in the United States, where clinical diphtheria has almost completely disappeared, isolation of toxigenic strains of C. diphtheriae is rare. Because subclinical infection no longer provides a source of exposure to diphtheria antigen and, unless boosted, immunity to antitoxin wanes, a large percentage of adults (30 to 60%) have antitoxin levels below protective levels and are at risk. In the United States, Europe, and Eastern Europe, recent outbreaks of diphtheria have occurred primarily among individuals who abuse alcohol and/or drugs. Within this group, carriers of toxigenic C. diphtheriae have a moderately high level of antitoxic immunity. The recent breakdown of public health measures in Russia has resulted in diphtheria becoming an epidemic. By the end of 1994, more than 80,000 cases and more than 2,000 deaths had been reported in Russia.
Focal outbreaks of diphtheria are almost always associated with an immune carrier who has returned from a region where diphtheria is endemic. Indeed, recent outbreaks of clinical diphtheria in the United States and Europe have been associated with travelers returning from Russia and Eastern Europe. Toxigenic strains of C. diphtheriae are transmitted directly from person to person by airborne droplets. It is known that toxigenic strains can directly colonize the nasopharyngeal cavity. In addition, the toxigenic gene can be spread indirectly by the release of toxigenic corynebacteriophage and lysogenic transformation of nontoxigenic autochthonous C. diphtheriae in situ. [ 5 ]
In addition to biotype and lysotype determination of C. diphtheriae isolates, molecular biology techniques can now be used to study diphtheria outbreaks. Restriction endonuclease digestion patterns of C. diphtheriae chromosomal DNA have been used to study clinical outbreaks, as has the use of cloned corynebacterial insertion sequences as a genetic probe.
The Schick test has been used for many years to assess immunity to diphtheria toxin, although it has now been replaced in many areas by the serologic test for specific antibodies to diphtheria toxin. In the Schick test, a small amount of diphtheria toxin (approximately 0.8 ng in 0.2 ml) is injected intradermally into the forearm (test site) and 0.0124 μg of diphtheria toxin in 0.2 ml is injected intradermally into the control site. Readings are taken at 48 and 96 hours. Nonspecific skin reactions usually peak at 48 hours. At 96 hours, an erythematous reaction with some possible necrosis at the test site indicates insufficient antitoxic immunity to neutralize the toxin (≤ 0.03 IU/ml). Inflammation in both the test and control areas after 48 hours indicates a hypersensitivity reaction to the antigen preparation.
Forms
Corynebacterium diphtheriae infects the nasopharynx or skin. Toxigenic strains produce a potent exotoxin that can cause diphtheria. Symptoms of diphtheria include pharyngitis, fever, and swelling of the neck or area around the skin lesion. Diphtheritic lesions are covered by a pseudomembrane. The toxin spreads through the bloodstream to distant organs and can cause paralysis and congestive heart failure. [ 6 ]
There are two types of clinical diphtheria: nasopharyngeal and cutaneous. Symptoms of pharyngeal diphtheria range from mild pharyngitis to hypoxia due to airway obstruction by a pseudomembrane. Involvement of the cervical lymph nodes may cause severe swelling of the neck (bull's neck diphtheria), and the patient may develop fever (≥ 103°F). Skin lesions in cutaneous diphtheria are usually covered by a gray-brown pseudomembrane. Life-threatening systemic complications, mainly loss of motor function (eg, difficulty swallowing) and congestive heart failure, may develop as a result of the action of diphtheria toxin on peripheral motor neurons and the myocardium.
Control
Control of diphtheria depends on adequate immunization with diphtheria toxoid: diphtheria toxin inactivated by formaldehyde, which remains antigenically intact. The toxoid is prepared by incubating diphtheria toxin with formaldehyde at 37°C under alkaline conditions. Diphtheria immunization should begin in the second month of life with a series of three primary doses at 4- to 8-week intervals, followed by a fourth dose approximately 1 year after the last primary. Diphtheria toxoid is widely used as a component of the diphtheria-pertussis-tetanus (DPT) vaccine. Epidemiological studies have shown that immunization against diphtheria is approximately 97% effective. Although mass immunization against diphtheria is practiced in the United States and Europe and childhood immunization rates are adequate, a large proportion of the adult population may have antibody titers below protective levels. Adults should be revaccinated with diphtheria toxoid every 10 years. Indeed, a booster immunization with diphtheria-tetanus toxoid should be given to travelers to areas with high rates of endemic diphtheria (Central and South America, Africa, Asia, Russia, and Eastern Europe). In recent years, the use of highly purified toxoid preparations for immunization has minimized occasional severe hypersensitivity reactions. a booster immunization with diphtheria-tetanus toxoid should be given to travelers to areas with high rates of endemic diphtheria (Central and South America, Africa, Asia, Russia, and Eastern Europe). In recent years, the use of highly purified toxoid preparations for immunization has minimized occasional severe hypersensitivity reactions. Booster immunization with diphtheria-tetanus toxoid should be given to travelers to areas with high rates of endemic diphtheria (Central and South America, Africa, Asia, Russia, and Eastern Europe). In recent years, the use of highly purified toxoid preparations for immunization has minimized occasional severe hypersensitivity reactions.
Although antibiotics (such as penicillin and erythromycin) are used as part of the treatment of patients with diphtheria, rapid passive immunization with diphtheria antitoxin is most effective in reducing case fatality. The long half-life of specific antitoxin in the bloodstream is an important factor in ensuring effective neutralization of diphtheria toxin; however, to be effective, the antitoxin must react with the toxin before it can enter the cell.
Reengineering of diphtheria toxin for the development of eukaryotic receptor-specific cytotoxins
Protein engineering is a new and rapidly developing field in molecular biology; it combines recombinant DNA methodologies and solid-phase DNA synthesis to design and construct chimeric genes whose products have unique properties. Studies of the structure-function relationships of diphtheria toxin have clearly shown that this toxin is a three-domain protein: catalytic, transmembrane, and receptor. It has been possible to genetically replace the native receptor-binding domain of diphtheria toxin with various polypeptide hormones and cytokines (e.g., α-melanocyte-stimulating hormone [α-MSH], interleukin (IL) 2, IL-4, IL-6, IL-7, epidermal growth factor). The resulting chimeric proteins or fusion toxins combine the receptor-binding specificity of the cytokine with the transmembrane and catalytic domains of the toxin. In each case, the fusion toxins were shown to selectively poison only those cells that bear the corresponding target receptor. The first of these genetically modified fusion toxins, DAB 389IL-2, is currently being evaluated in human clinical trials for the treatment of refractory lymphomas and autoimmune diseases in which cells with high-affinity IL-2 receptors play an important role in pathogenesis.[ 7 ] Administration of DAB 389 IL-2 was shown to be safe, well tolerated, and capable of inducing durable disease remission with no serious side effects. It is likely that diphtheria toxin-based fusion toxins will become important new biologic agents for the treatment of specific tumors or diseases in which specific cell surface receptors can be targeted.


 [
[