Medical expert of the article
New publications
Hereditary nephritis (Alport syndrome) in children
Last reviewed: 05.07.2025

All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.
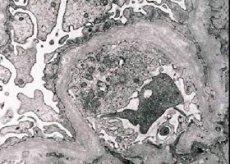
Hereditary nephritis (Alport syndrome) is a genetically determined hereditary non-immune glomerulopathy, manifested by hematuria (sometimes with proteinuria), progressive decline in renal function with the development of chronic renal failure, often combined with sensorineural deafness and visual impairment.
The disease was first described in 1902 by L. G. Guthrie, who observed a family in which hematuria was observed in several generations. In 1915, A. F. Hurst described the development of uremia in members of the same family. In 1927, A. Alport first identified hearing loss in several relatives with hematuria. In the 1950s, eye lesions in a similar disease were described. In 1972, in patients with hereditary hematuria, during a morphological study of renal tissue, Hinglais et al. revealed uneven expansion and stratification of the glomerular basement membranes. In 1985, the genetic basis of hereditary nephritis was identified - a mutation in the type IV collagen gene (Fiengold et al., 1985).
The study of the genetic nature of the disease allowed us to conclude that the differences in the phenotypic manifestations of hereditary nephritis (with or without hearing loss) are due to the degree of expression of the mutant gene. Thus, at present, all clinical variants are considered as manifestations of one disease and the term "hereditary nephritis" is synonymous with the term "Alport syndrome".
According to epidemiological studies, hereditary nephritis occurs with a frequency of 17 per 100,000 children.
Causes of Alport Syndrome
The genetic basis of the disease is a mutation in the gene of the a-5 chain of type IV collagen. This type is universal for the basal membranes of the kidney, cochlear apparatus, lens capsule, retina and cornea of the eye, which has been proven in studies using monoclonal antibodies against this collagen fraction. Recently, the possibility of using DNA probes for prenatal diagnostics of hereditary nephritis has been indicated.
The importance of testing all family members with DNA probes to identify carriers of the mutant gene is emphasized, which is of great importance in conducting medical and genetic counseling of families with this disease. However, up to 20% of families do not have relatives suffering from kidney disease, which suggests a high frequency of spontaneous mutations of the abnormal gene. Most patients with hereditary nephritis have individuals with kidney disease, hearing loss, and vision pathology in their families; consanguineous marriages between people with one or more ancestors are important, since in the marriage of related individuals the probability of receiving the same genes from both parents increases. Autosomal dominant, autosomal recessive, and dominant, X-linked transmission routes have been established.
In children, three types of hereditary nephritis are most commonly distinguished: Alport syndrome, hereditary nephritis without hearing loss, and familial benign hematuria.
Alport syndrome is a hereditary nephritis with hearing impairment. It is based on a combined defect in the structure of the collagen of the glomerular basement membrane of the kidneys, ear and eye structures. The gene of classical Alport syndrome is located in the locus 21-22 q of the long arm of the X chromosome. In most cases, it is inherited in a dominant manner, linked to the X chromosome. In this regard, Alport syndrome is more severe in men, since in women the function of the mutant gene is compensated by a healthy allele of the second, undamaged chromosome.
The genetic basis for the development of hereditary nephritis is mutations in the genes of the alpha chains of type IV collagen. Six alpha chains of type IV collagen G are known: the genes of the a5- and a6-chains (Col4A5 and Col4A5) are located on the long arm of the X chromosome in the 21-22q zone; the genes of the a3- and a4-chains (Col4A3 and Col4A4) are on the 2nd chromosome; the genes of the a1- and a2-chains (Col4A1 and Col4A2) are on the 13th chromosome.
In most cases (80-85%), an X-linked inheritance pattern of the disease is detected, associated with damage to the Col4A5 gene as a result of deletion, point mutations or splicing disorders. Currently, more than 200 mutations of the Col4A5 gene have been found, responsible for the disruption of the synthesis of the a5-chains of type IV collagen. With this type of inheritance, the disease manifests itself in children of both sexes, but in boys it is more severe.
Mutations in the loci of the Col4A3 and Col4A4 genes responsible for the synthesis of the a3 and a4 chains of type IV collagen are inherited autosomal. According to research, the autosomal dominant type of inheritance is observed in 16% of cases of hereditary nephritis, and the autosomal recessive type is observed in 6% of patients. About 10 variants of mutations of the Col4A3 and Col4A4 genes are known.
The result of mutations is a violation of the assembly processes of type IV collagen, leading to a violation of its structure. Type IV collagen is one of the main components of the glomerular basement membrane, cochlear apparatus and lens of the eye, the pathology of which will be detected in the clinic of hereditary nephritis.
Collagen type IV, which is part of the glomerular basement membrane, consists mainly of two a1-chains (IV) and one a2-chain (IV), and also contains a3, a4, a5-chains. Most often, in X-linked inheritance, the mutation of the Col4A5 gene is accompanied by the absence of a3-, a4-, a5- and a6-chains in the structure of collagen type IV, and the number of o1- and a2-chains in the glomerular basement membrane increases. The mechanism of this phenomenon is unclear, it is assumed that the cause is post-transcriptional changes in mRNA.
The absence of a3, a4, and a5 chains in the structure of type IV collagen of the glomerular basement membranes leads to their thinning and fragility in the early stages of Alport syndrome, which is clinically manifested more often by hematuria (less often by hematuria with proteinuria or only proteinuria), hearing loss, and lenticonus. Further progression of the disease leads to thickening and impaired permeability of the basement membranes in the late stages of the disease, with the proliferation of collagen types V and VI in them, manifested in an increase in proteinuria and a decrease in renal function.
The nature of the mutation underlying hereditary nephritis largely determines its phenotypic manifestation. In the case of deletion of the X chromosome with simultaneous mutation of the Col4A5 and Col4A6 genes responsible for the synthesis of the a5- and a6-chains of type IV collagen, Alport syndrome is combined with leiomyomatosis of the esophagus and genitals. According to research data, in the case of a mutation of the Col4A5 gene associated with a deletion, a greater severity of the pathological process is noted, a combination of renal damage with extrarenal manifestations and early development of chronic renal failure, compared to a point mutation of this gene.
Morphologically, electron microscopy reveals thinning and stratification of the glomerular basement membranes (especially lamina densa) and the presence of electron-dense granules. Glomerular lesions may be heterogeneous in the same patient, from minimal focal mesangial lesions to glomerulosclerosis. Glomerulitis in Alport syndrome is always immunonegative, which distinguishes it from glomerulonephritis. Characteristic features include the development of tubular atrophy, lymphohistiocytic infiltration, and the presence of "foam cells" with lipid inclusions - lipophages. As the disease progresses, thickening and pronounced destruction of the glomerular basement membranes are revealed.
Certain changes in the immune system are revealed. Patients with hereditary nephritis have a decreased Ig A level and a tendency to increase IgM concentration in the blood, the IgG level may be increased in the early stages of the disease and decrease in the later stages. Perhaps, the increase in IgM and G concentration is a kind of compensatory reaction in response to IgA deficiency.
The functional activity of the T-lymphocyte system is reduced; a selective decrease in B-lymphocytes responsible for the synthesis of Ig A is noted, the phagocytic link of immunity is disrupted, mainly due to disruption of chemotaxis and intracellular digestion processes in neutrophils
When examining a kidney biopsy in patients with Alport syndrome, electron microscopy data reveals ultrastructural changes in the glomerular basement membrane: thinning, disruption of the structure and splitting of the glomerular basement membranes with a change in its thickness and uneven contours. In the early stages of hereditary nephritis, the defect determines the thinning and fragility of the glomerular basement membranes.
Thinning of the glomerular membranes is a more favorable sign and is more common in girls. A more constant electron microscopic sign in hereditary nephritis is the splitting of the basement membrane, and the severity of its destruction correlates with the severity of the process.
Symptoms of Alport Syndrome in Children
The first symptoms of Alport syndrome in the form of isolated urinary syndrome are most often detected in children of the first three years of life. In most cases, the disease is detected by chance. Urinary syndrome is detected during a preventive examination of the child, before admission to a child care facility or during ARVI. In the event of pathology in the urine during ARVI. In hereditary nephritis, unlike acquired glomerulonephritis, there is no latent period.
At the initial stage of the disease, the child's health suffers little, a characteristic feature is the persistence and resistance of the urinary syndrome. One of the main signs is hematuria of varying degrees of severity, observed in 100% of cases. An increase in the degree of hematuria is noted during or after respiratory infections, physical activity or after preventive vaccinations. Proteinuria in most cases does not exceed 1 g / day, at the beginning of the disease can be inconstant, as the process progresses, proteinuria increases. Periodically, leukocyturia with a predominance of lymphocytes may be present in the urinary sediment, which is associated with the development of interstitial changes.
Subsequently, partial renal function is impaired, the patient's general condition worsens: intoxication, muscle weakness, arterial hypotension, often hearing impairment (especially in boys), and sometimes visual impairment appear. Intoxication is manifested by pallor, fatigue, and headaches. In the initial stage of the disease, hearing loss is in most cases detected only by audiography. Hearing loss in Alport syndrome can occur at different periods of childhood, but most often hearing loss is diagnosed at the age of 6-10 years. Hearing loss in children begins with high frequencies, reaching a significant degree in air and bone conduction, passing from sound-conducting to sound-perceiving hearing loss. Hearing loss can be one of the first symptoms of the disease and can precede urinary syndrome.
In 20% of cases, patients with Alport syndrome have changes in the visual organs. The most frequently detected anomalies are those of the lens: spherophokia, anterior, posterior or mixed lenticonus, and various cataracts. In families with Alport syndrome, there is a significant frequency of myopia. A number of researchers constantly note bilateral perimacular changes in these families in the form of bright whitish or yellowish granulations in the corpus luteum. They consider this sign to be a constant symptom that has high diagnostic value in Alport syndrome. K. S. Chugh et al. (1993) in an ophthalmological study found in patients with Alport syndrome a decrease in visual acuity in 66.7% of cases, anterior lenticonus in 37.8%, retinal spots in 22.2%, cataracts in 20%, and keratoconus in 6.7%.
In some children with hereditary nephritis, especially when renal failure develops, a significant lag in physical development is noted. As renal failure progresses, arterial hypertension develops. In children, it is more often detected in adolescence and in older age groups.
Patients with hereditary nephritis are characterized by the presence of various (more than 5-7) stigmas of connective tissue dysmorphogenesis. Among the connective tissue stigmas in patients, the most common are hypertelorism of the eyes, high palate, bite anomalies, abnormal shape of the auricles, curvature of the little finger on the hands, and "sandal gap" on the feet. Hereditary nephritis is characterized by the uniformity of dysmorphogenesis stigmas within a family, as well as a high frequency of their distribution among relatives of probands along whose line the disease is transmitted.
In the early stages of the disease, an isolated decrease in partial renal functions is detected: transport of amino acids, electrolytes, concentration function, acidogenesis, later changes affect the functional state of both the proximal and distal parts of the nephron and are characterized by combined partial disorders. A decrease in glomerular filtration occurs later, more often in adolescence. As hereditary nephritis progresses, anemia develops.
Thus, hereditary nephritis is characterized by a staged course of the disease: first, a latent stage or hidden clinical symptoms, manifested by minimal changes in the urinary syndrome, then a gradual decompensation of the process occurs with a decrease in renal function with manifest clinical symptoms (intoxication, asthenia, developmental delay, anemia). Clinical symptoms usually appear regardless of the layering of the inflammatory reaction.
Hereditary nephritis can manifest itself at different age periods, which depends on the action of the gene, which is in a repressed state until a certain time.
Classification
There are three types of hereditary nephritis
- Option I - clinically manifests as nephritis with hematuria, hearing loss and eye damage. The course of nephritis is progressive with the development of chronic renal failure. The type of inheritance is dominant, linked to the X chromosome. Morphologically, a violation of the structure of the basement membrane, its thinning and splitting are revealed.
- Option II - clinically manifests as nephritis with hematuria without hearing loss. The course of nephritis is progressive with the development of chronic renal failure. The type of inheritance is dominant, linked to the X chromosome. Morphologically, thinning of the glomerular capillary basement membrane (especially laminadensa) is detected.
- Option III - benign familial hematuria. The course is favorable, chronic renal failure does not develop. The type of inheritance is autosomal dominant or autosomal recessive. With the autosomal recessive type of inheritance, a more severe course of the disease is noted in women.
Diagnosis of Alport syndrome
The following criteria are proposed:
- the presence of at least two patients with nephropathy in each family;
- hematuria as the leading symptom of nephropathy in the proband;
- the presence of hearing loss in at least one family member;
- development of chronic renal failure in one or more relatives.
In diagnostics of various hereditary and congenital diseases, a large place is given to a comprehensive approach to examination and, above all, paying attention to the data obtained when compiling the child's pedigree. The diagnosis of Alport syndrome is considered valid in cases where 3 of 4 typical signs are detected in the patient: the presence of hematuria and chronic renal failure in the family, the presence of neurosensory hearing loss, vision pathology in the patient, detection of signs of cleavage of the glomerular basement membrane with a change in its thickness and uneven contours during electron microscopic characteristics of the biopsy.
The examination of the patient should include clinical and genetic research methods; targeted study of the disease history; general examination of the patient taking into account diagnostically significant criteria. In the compensation stage, pathology can be detected only by focusing on such syndromes as the presence of a hereditary burden, hypotension, multiple stigmas of dysembryogenesis, changes in the urinary syndrome. In the decompensation stage, extrarenal symptoms may appear, such as severe intoxication, asthenia, delayed physical development, anemia, manifesting and intensifying with a gradual decrease in renal function. In most patients, with a decrease in renal function, the following is observed: decreased acido- and aminogenesis; 50% of patients note a significant decrease in the secretory function of the kidneys; limited range of fluctuations in the optical density of urine; disturbance of the filtration rhythm, and then a decrease in glomerular filtration. The stage of chronic renal failure is diagnosed when patients have an elevated level of urea in the blood serum (more than 0.35 g/l) for 3-6 months or more, and a decrease in glomerular filtration to 25% of the norm.
Differential diagnostics of hereditary nephritis should be performed primarily with the hematuric form of acquired glomerulonephritis. Acquired glomerulonephritis most often has an acute onset, a period of 2-3 weeks after an infection, extrarenal signs, including hypertension from the first days (in hereditary nephritis, on the contrary, hypotension), decreased glomerular filtration at the onset of the disease, no impairment of partial tubular functions, whereas in hereditary they are present. Acquired glomerulonephritis occurs with more pronounced hematuria and proteinuria, with an increased ESR. Typical changes in the glomerular basement membrane, characteristic of hereditary nephritis, are of diagnostic value.
Differential diagnostics from dysmetabolic nephropathy is carried out with chronic renal failure, in the family clinically revealed heterogeneous kidney diseases, and there may be a spectrum of nephropathy from pyelonephritis to urolithiasis. Children often have complaints of pain in the abdomen and periodically during urination, in the urine sediment - oxalates.
If hereditary nephritis is suspected, the patient should be referred to a specialized nephrology department to clarify the diagnosis.
What do need to examine?
How to examine?
What tests are needed?
Who to contact?
Treatment of Alport syndrome
The regimen includes restrictions on heavy physical exertion and exposure to fresh air. The diet is complete, with sufficient levels of complete proteins, fats and carbohydrates, taking into account kidney function. Of great importance is the detection and treatment of chronic foci of infection. The following medications are used: ATP, cocarboxylase, pyridoxine (up to 50 mg/day), carnitine chloride. Courses are administered 2-3 times a year. For hematuria, herbal medicine is prescribed - stinging nettle, chokeberry juice, yarrow.
There are reports in foreign and domestic literature about treatment with prednisolone and the use of cytostatics. However, it is difficult to judge the effect.
In chronic renal failure, hemodialysis and kidney transplantation are used.
There are no methods of specific (effective pathogenetic) therapy for hereditary nephritis. All treatment measures are aimed at preventing and slowing down the decline in renal function.
The diet should be balanced and high-calorie, taking into account the functional state of the kidneys. In the absence of functional disorders, the child's diet should contain sufficient proteins, fats and carbohydrates. In the presence of signs of renal dysfunction, the amount of protein, carbohydrates, calcium and phosphorus should be limited, which delays the development of chronic renal failure.
Physical activity should be limited; children are advised to avoid sports.
Contact with infectious patients should be avoided, the risk of developing acute respiratory diseases should be reduced. Sanitation of foci of chronic infection is necessary. Preventive vaccinations are not carried out for children with hereditary nephritis, vaccination is possible only for epidemiological indications.
Hormonal and immunosuppressive therapy in hereditary nephritis is ineffective. There are indications of some positive effect (reduction in proteinuria and slowing of disease progression) with long-term multi-year use of cyclosporine A and ACE inhibitors.
In the treatment of patients, drugs are used that improve metabolism:
- pyridoxine - 2-3 mg/kg/day in 3 doses for 4 weeks;
- cocarboxylase - 50 mg intramuscularly every other day, a total of 10-15 injections;
- ATP - 1 ml intramuscularly every other day, 10-15 injections;
- vitamin A - 1000 IU/year/day in 1 dose for 2 weeks;
- Vitamin E - 1 mg/kg/day in 1 dose for 2 weeks.
This type of therapy helps improve the general condition of patients, reduce tubular dysfunctions and is carried out in courses 3 times a year.
Levamisole can be used as an immunomodulator - 2 mg/kg/day 2-3 times a week with breaks between doses of 3-4 days.
According to research data, hyperbaric oxygenation has a positive effect on the severity of hematuria and renal dysfunction.
The most effective method of treating hereditary nephritis is timely kidney transplantation. In this case, there is no relapse of the disease in the transplant; in a small percentage of cases (about 5%), nephritis may develop in the transplanted kidney associated with antigens to the glomerular basement membrane.
A promising direction is prenatal diagnostics and genetic engineering therapy. Animal experiments show high efficiency of transferring normal genes responsible for the synthesis of type IV collagen alpha chains into renal tissue, after which the synthesis of normal collagen structures is observed.
Forecast
The prognosis for hereditary nephritis is always serious.
Prognostically unfavorable criteria for the course of hereditary nephritis are:
- male gender;
- early development of chronic renal failure in family members;
- proteinuria (more than 1 g/day);
- thickening of the glomerular basement membranes according to microscopy;
- acoustic neuritis;
- deletion in the Col4A5 gene.
The prognosis for benign familial hematuria is more favorable.
Использованная литература


 [
[