New publications
Sleep cleanses the brain of toxins and metabolites
Last reviewed: 02.07.2025

All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.

A recent study published in the journal Nature Neuroscience found that brain clearing is reduced during anesthesia and sleep.
Sleep is a state of vulnerable inactivity. Given the risks of this vulnerability, it has been suggested that sleep may confer some benefits. It has been suggested that sleep clears toxins and metabolites from the brain via the glymphatic system. This suggestion has important implications; for example, reduced clearance of toxins due to chronically poor sleep may worsen Alzheimer's disease.
The mechanisms and anatomical pathways by which toxins and metabolites are cleared from the brain remain unclear. According to the glymphatic hypothesis, the basal fluid flow, driven by hydrostatic pressure gradients from arterial pulsations, actively clears salts from the brain during slow wave sleep. In addition, sedative doses of anesthetics enhance clearance. Whether sleep enhances clearance through increased basal flow remains unknown.
In this study, the researchers measured fluid movement and brain clearance in mice. First, they determined the diffusion coefficient of fluorescein isothiocyanate (FITC)-dextran, a fluorescent dye. FITC-dextran was injected into the caudate nucleus, and fluorescence was measured in the frontal cortex.
Initial experiments involved waiting for steady state, bleaching the dye in a small volume of tissue, and determining the diffusion coefficient by measuring the rate of movement of unbleached dye into the bleached area. The technique was validated by measuring the diffusion of FITC-dextran in brain-simulating agarose gels that were modified to approximate the optical absorption and light scattering of the brain.
The results showed that the diffusion coefficient of FITC-dextran did not differ between the anesthetized and sleep states. The team then measured brain clearance in different states of wakefulness. They used a small volume of the fluorescent dye AF488 in mice that were injected with saline or anesthetic. This dye moved freely in the parenchyma and could help accurately quantify brain clearance. Comparisons were also made between the awake and sleep states.
At peak concentrations, clearance was 70–80% in saline-treated mice, indicating that normal clearance mechanisms were not impaired. However, clearance was significantly reduced when anesthetics (pentobarbital, dexmedetomidine, and ketamine-xylazine) were used. In addition, clearance was also reduced in sleeping mice compared to awake mice. However, the diffusion coefficient was not significantly different between the anesthetized and sleeping states.
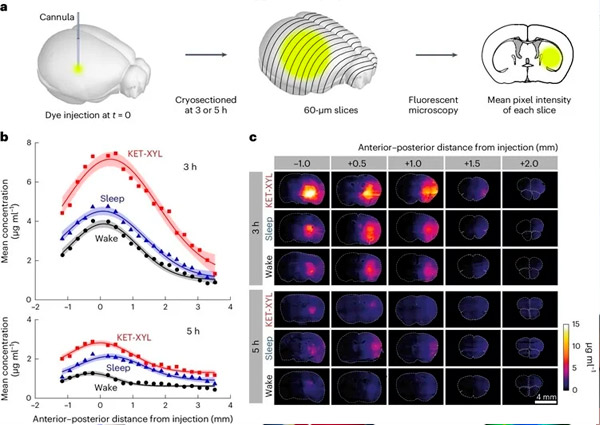
A. Three or five hours after AF488 injection into CPu, the brains were frozen and cut into 60-μm-thick cryosections. The mean fluorescence intensity of each section was measured by fluorescence microscopy; the mean intensities of groups of four sections were then averaged.
B. Mean fluorescence intensity was converted to concentration using the calibration data presented in Supplementary Figure 1 and plotted against anteroposterior distance from the injection point for awake (black), asleep (blue), and KET-XYL anesthesia (red) states. Top is data at 3 hours. Bottom is data at 5 hours. Lines represent Gaussian fits to the data, and error bars show 95% confidence intervals. At both 3 and 5 h, KET-XYL concentrations during anesthesia (P < 10⁻⁶ at 3 h; P < 10⁻⁶ at 5 h) and sleep (P = 0.0016 at 3 h; P < 10⁻⁴ at 5 h) were significantly higher than those during wakefulness (two-way ANOVA with Bonferroni–Holm multiple comparison correction).
C. Representative images of brain sections at different distances (anteroposterior) from the AF488 injection site after 3 hours (upper three rows) and after 5 hours (lower three rows). Each row represents data for three waking states (awake, sleep, and KET-XYL anesthesia).
The study found that brain clearance was reduced during anesthesia and sleep, which contradicts previous reports. Clearance may vary across anatomical sites, but the degree of variation may be small. However, the inhibition of clearance by ketamine-xylazine was significant and independent of site.
Nicholas P. Franks, one of the study's authors, said: "The research field has been so focused on the idea of cleaning as one of the key reasons why we sleep that we were very surprised by the opposite results."
It is particularly important to note that the results concern a small volume of dye that moves freely in the extracellular space. Larger molecules may exhibit different behavior. In addition, the precise mechanisms by which sleep and anesthesia affect brain clearance remain unclear; however, these findings challenge the notion that the primary function of sleep is to clear the brain of toxins.
