New publications
New study explains weakened immune response in older adults
Last reviewed: 02.07.2025

All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.
A fully functioning immune system is essential to maintaining the body's health, and macrophages play a critical role in maintaining strong immune responses against infections.
A macrophage is a type of white blood cell that destroys microorganisms, removes dead cells, and stimulates the action of other immune cells. These cells play a key role in initiating, maintaining, and resolving inflammation, but their functionality declines with age, leading to a decline in immune function. This leads to increased susceptibility to infections and autoimmune diseases in the elderly population.
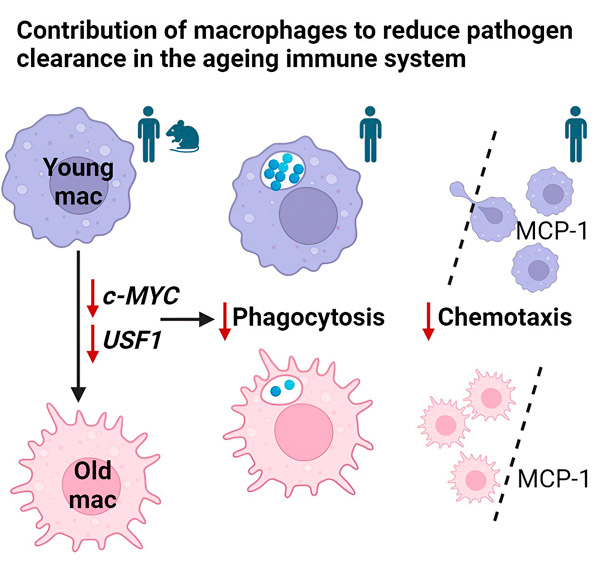
A study published in the journal Cell Reports is the first to reveal that defects in macrophage function are caused by the MYC and USF1 transcriptional programs.
Research led by Charlotte Moss, Dr Heather Wilson and Professor Endre Kiss-Toth has identified a possible culprit for this decline: two critical molecules inside macrophages, MYC and USF1, which start to malfunction as we age.
Macrophages, often referred to as the body's "garbage trucks," are responsible for engulfing and eliminating foreign particles, including debris and pathogens. A study found that macrophages isolated from older adults were significantly less effective than those from younger adults. These aging macrophages showed decreased phagocytosis (the process of engulfing foreign particles) and impaired chemotaxis (the ability to migrate toward threats).
Confirming this link, the researchers artificially reduced the activity of MYC and USF1 in young macrophages. This manipulation resulted in functional decline that resembled the characteristics of macrophages from older adults. This finding strongly suggests that MYC and USF1 play important roles in maintaining optimal macrophage function.
The study goes beyond simply identifying the culprits. It examines how decreased activity of MYC and USF1 might affect macrophages. The researchers speculate that these changes might disrupt genes responsible for the cell’s internal cytoskeleton, a network of threads that provides structure and movement.
This disruption may interfere with the macrophage’s ability to move and engulf foreign particles. Additionally, altered MYC and USF1 activity may affect how macrophages interact with their environment, further impairing their ability to fight infection.
This study represents a significant breakthrough in understanding the mechanisms of age-related immune decline.
Graphical figure. Source: Cell Reports (2024). DOI: 10.1016/j.celrep.2024.114073
By identifying MYC and USF1 as potential culprits, the study paves the way for the development of new therapeutic strategies. By targeting these molecules or their gene products, researchers may be able to improve macrophage function in older adults, which could lead to a stronger immune response and improved resistance to infections.
It is important to note that the study included healthy volunteers and did not include people with pre-existing age-related diseases. In addition, the studies were conducted in a controlled laboratory setting. Further studies are needed to confirm these findings in a larger population and to explore the possibility of translating these findings into effective therapies.
The identification of MYC and USF1 as potential targets for intervention represents a significant step forward. This study opens up prospects for the development of future strategies to strengthen the immune system in older adults, ultimately promoting healthier aging.
"Understanding why the immune system stops fighting infections effectively in old age is key to developing treatments that can reverse this process. Our work reveals for the first time the molecular details of aging in human phagocytes, and we believe that this new understanding now allows us to test the effectiveness of various interventions, including diet, lifestyle and even potential drugs aimed at reversing the aging of the immune system," says Endre Kiss-Toth.
