New publications
Scientists track the earliest physical changes in cells that cause cancer
Last reviewed: 02.07.2025

All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.
When cancer is diagnosed, there are already many events at the cellular and molecular levels that have been going on unnoticed. Although cancer is classified into early and late stages for clinical purposes, even an "early" stage tumor is the result of many previous changes in the body that were undetectable.
Now, scientists at Yale University School of Medicine (YSM) and their colleagues have gained detailed insight into some of these early changes, using powerful high-resolution microscopy to track the very first cancer-causing physical changes in mouse skin cells.
By studying mice carrying a mutation that promotes cancer in their hair follicles, the scientists found that the first signs of cancer formation occur at a specific time and place in the growth of the mice's hair follicles. What's more, they found that these precancerous changes can be blocked with drugs known as MEK inhibitors.
The team was led by Tianchi Xin, PhD, a research associate in the YSM Department of Genetics, and included Valentina Greco, PhD, a professor of genetics at YSM and a member of the Yale Cancer Center and the Yale Stem Cell Center, and Sergi Regot, PhD, an associate professor of molecular biology and genetics at the Johns Hopkins School of Medicine.
The results of their research were published in the journal Nature Cell Biology.
The scientists studied mice that develop cutaneous squamous cell carcinoma, the second most common type of skin cancer in humans. These mice were genetically modified with a cancer-promoting mutation in the KRAS gene, which is one of the most commonly mutated oncogenes in human cancers. KRAS mutations have also been found in lung, pancreatic, and colorectal cancers.
The early changes the scientists studied included the growth of a tiny, abnormal bump in the hair follicle, which is classified as a precancerous abnormality. "Understanding these early events may help us develop approaches to prevent cancer from eventually forming," said Xin, the study's first author.
Although their study focuses on skin cancer, the researchers believe the principles they discovered could be applied to many other cancers caused by KRAS mutations because the key genes and proteins involved in these processes are the same across tumors.
More than just cell proliferation In both humans and mice, hair follicles are constantly growing, shedding old hair and forming new ones. Stem cells, which have the ability to develop into different cell types, play a large role in this renewal process. Previous studies have shown that KRAS mutations lead to increased stem cell proliferation in hair follicles, and this large increase in stem cells was thought to be responsible for the precancerous tissue disorder.
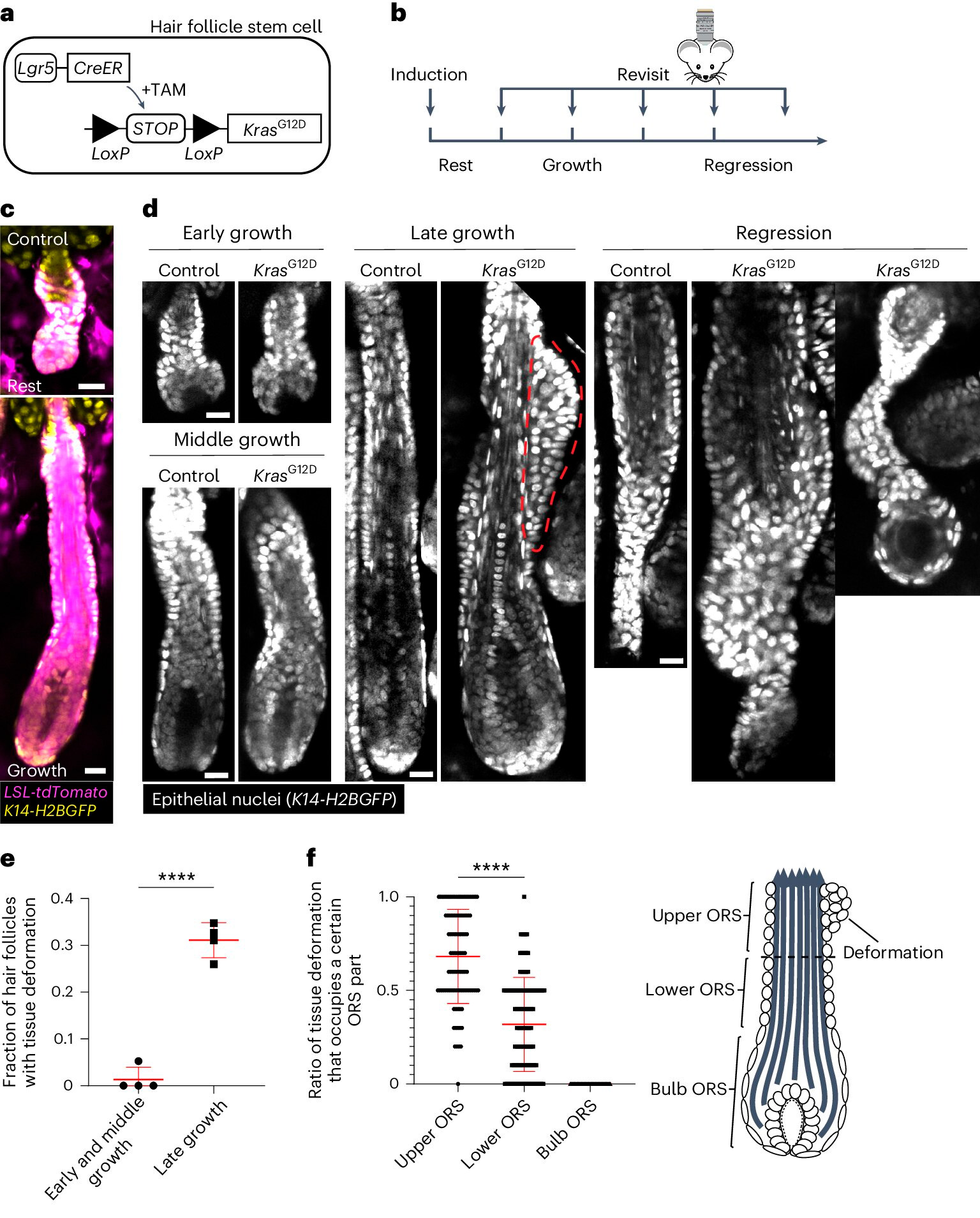
KrasG12D induces spatiotemporal specific tissue deformations during hair follicle regeneration.
A. Schematic of the genetic approach to induce KrasG12D in hair follicle stem cells using the tamoxifen-inducible Cre–LoxP (TAM) system.
B. Schematic showing the timing of KrasG12D induction and re-imaging relative to hair cycle stages.
C. Representative images of wild-type quiescent and growing hair follicles containing the Cre-inducible tdTomato (Magenta) reporter after induction.
D. Representative images of control and KrasG12D hair follicles at different hair cycle stages. Tissue deformation as tubercles in the outer root sheath (ORS) is indicated by the red dotted line.
E. Proportion of KrasG12D hair follicles with tissue deformation at different stages of hair follicle growth.
F. Proportion of tissue deformations occupying the upper, lower, and bulbous parts of the ORS for individual KrasG12D hair follicles.
Source: Nature Cell Biology (2024). DOI: 10.1038/s41556-024-01413-y
To test this idea, the team used a specially engineered form of mutated KRAS that they could activate at specific times in the skin cells of the animals’ hair follicles. Xin and his colleagues used a microscopy technique known as intravital imaging, which allows high-resolution images of cells to be taken in vivo, and to tag and track individual stem cells in the animals.
When the KRAS mutation was activated, all the stem cells began to proliferate faster, but the precancerous bump formed only in one specific location in the hair follicle and at one stage of growth, meaning that the overall increase in cell numbers was likely not the whole story.
Activation of the KRAS mutation in hair follicles resulted in stem cells proliferating more rapidly, changing their migration patterns and dividing in different directions compared to cells without the cancer-promoting mutation.
The mutation affects a protein known as ERK. Xin was able to monitor ERK activity in real time in individual stem cells in living animals and found a specific change in the activity of this protein caused by the KRAS mutation. The researchers were also able to stop the formation of the precancerous lump using a MEK inhibitor, which blocks ERK activity.
The drug stopped the mutation's effects on cell migration and orientation, but not on overall stem cell proliferation, meaning that the formation of the precancerous condition is driven by these first two changes, rather than increased cell proliferation.
Precancerous Changes in Context Tracking the effects of an oncogenic mutation in real time in a living organism is the only way the researchers have been able to uncover these principles. This is important because cancers don’t form in a vacuum — they rely heavily on their microenvironment to grow and maintain themselves. The scientists also needed to track not just the behavior of individual cells, but also the molecules inside those cells.
"The approach we've taken to understanding these oncogenic events is really about connecting across scales," Greco said. "The structure and approaches that Dr. Xin and Dr. Regot have used have allowed us to go down to the molecular elements, connecting them to the cellular and tissue scale, giving us a resolution of these events that is so difficult to achieve outside of a living organism."
The researchers now want to follow the process over a longer period of time to see what happens after the initial bulge forms. They also want to study other oncogenic events, such as inflammation, to see if the principles they have discovered apply in other contexts.
