New publications
Scientists discover new immunosuppressive mechanism in brain cancer
Last reviewed: 02.07.2025

All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.
Associate Professor Filippo Veglia, Ph.D., and his team at the Wistar Institute have discovered a key mechanism by which glioblastoma – a serious and often fatal brain cancer – suppresses the immune system so that the tumor can grow undeterred by the body's defenses.
Their discovery was published in the article "Glucose-driven histone lactylation promotes the immunosuppressive activity of monocyte-derived macrophages in glioblastoma" in the journal Immunity.
"Our study shows that cancer's self-perpetuation mechanisms, if sufficiently understood, can be harnessed against the disease very effectively," said Dr. Veglia.
"I look forward to future studies of metabolism-mediated immunosuppression mechanisms in glioblastoma and hope that we will continue to learn more about how to better understand and fight this cancer."
Until now, little has been studied how monocyte-derived macrophages and microglia create an immunosuppressive tumor microenvironment in glioblastoma.
The Veglia lab investigated the cellular mechanisms of immunosuppression in glioblastoma and found that as glioblastoma progresses, monocyte-derived macrophages begin to outnumber microglia, suggesting that the predominance of monocyte-derived macrophages in the tumor microenvironment is beneficial to cancer in terms of immune evasion.
Indeed, monocyte-derived macrophages, but not microglia, blocked the activity of T cells (immune cells that kill tumor cells) in preclinical models and in patients. The team confirmed this by evaluating preclinical glioblastoma models with artificially reduced numbers of monocyte-derived macrophages.
As expected, models with fewer malignant macrophages in the tumor microenvironment showed improved results compared to standard glioblastoma models.
Glioblastoma accounts for just over half of all malignant tumors that arise in the brain, and the prognosis for patients diagnosed with the disease is extremely poor: only 25% of patients survive the first year after diagnosis. Glioblastoma is dangerous not only because of its location in the brain, but also because of the immunosuppressive tumor microenvironment, which makes glioblastoma resistant to promising immunotherapies.
By programming certain immune cells, such as macrophages (monocyte-derived macrophages and microglia), to work for the tumor rather than against it, glioblastoma creates a tumor microenvironment for itself that allows the cancer to grow aggressively while evading anti-cancer immune responses.
Clarification of the mechanism
Having confirmed the role of monocyte-derived macrophages, Weglia's lab then sought to understand exactly how these cancer-associated immune cells work against the immune system.
They sequenced the macrophages to determine whether the cells had any abnormal gene expression patterns that might indicate genes that play a role in immunosuppression, and they also examined the macrophages' metabolic patterns to see whether the abnormal gene expression was related to metabolism.
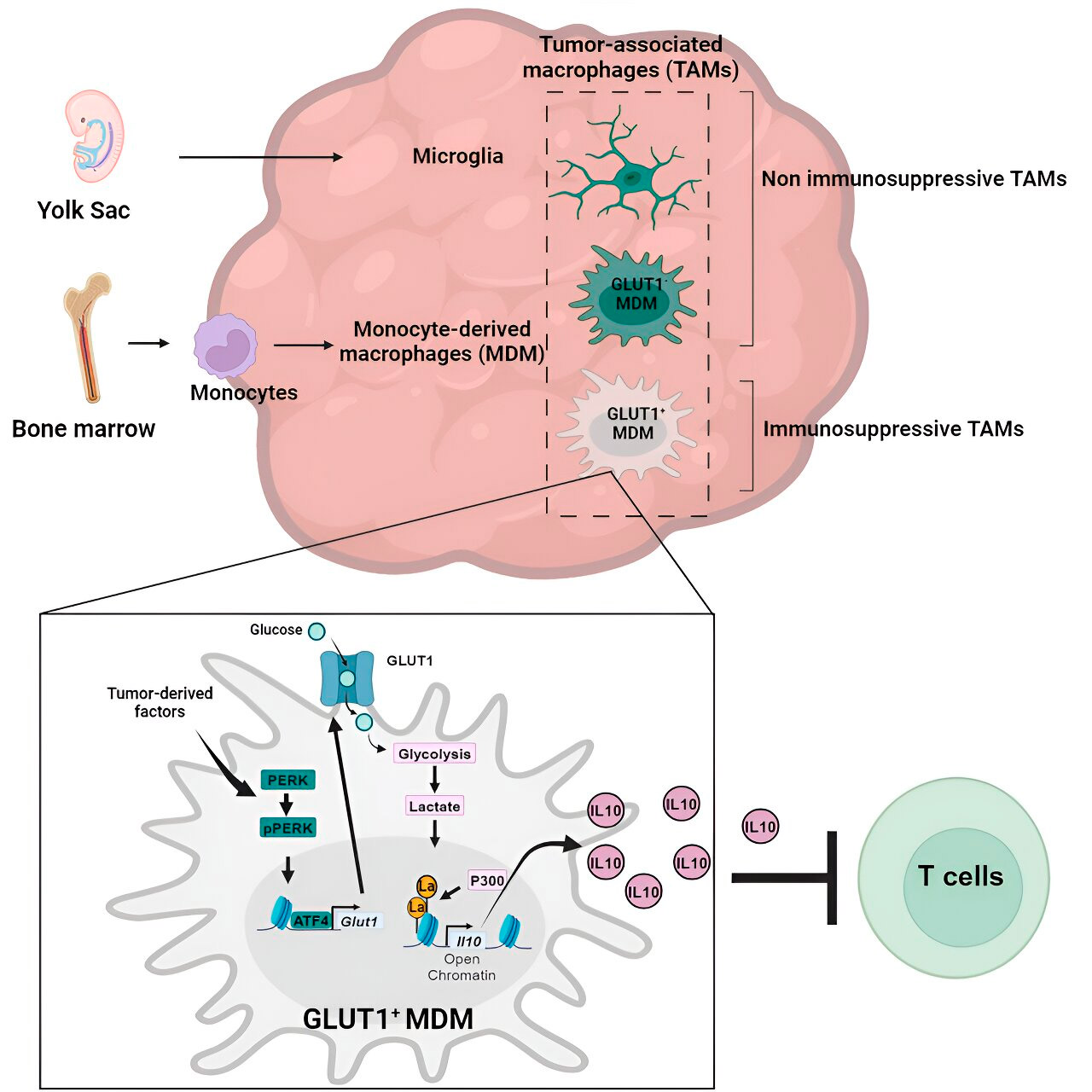
Gene and metabolic analysis led them to glucose metabolism. A series of tests showed that monocyte-derived macrophages with increased glucose metabolism and expression of GLUT1, the main transporter for glucose, blocked T-cell function by releasing interleukin-10 (IL-10).
The team demonstrated that glioblastoma disrupts glucose metabolism in these macrophages, causing their immunosuppressive activity.
Histone lactylation and its role
The researchers found that the key to the glucose metabolism-related immunosuppressive activity of monocyte-derived macrophages lies in a process called "histone lactylation." Histones are structural proteins in the genome that play a key role in the expression of genes such as IL-10 in certain contexts.
By rapidly metabolizing glucose, monocyte-derived macrophages produce lactate, a byproduct of glucose metabolism. Histones can become "lactylated" (i.e., lactate is integrated into the histones) in such a way that the histone organization promotes the expression of IL-10, which is produced by monocyte-derived macrophages to support cancer cell growth.
Solution to the problem
But how can the immunosuppressive activity of monocyte-derived macrophages associated with glucose metabolism be stopped? Dr. Veglia and his team identified a possible solution: PERK, an enzyme they identified as a regulator of glucose metabolism and GLUT1 expression in macrophages.
In preclinical models of glioblastoma, targeting PERK impaired histone lactylation and macrophage immunosuppressive activity, and when combined with immunotherapy, blocked glioblastoma progression and induced long-term immunity that protected the brain from tumor regrowth, suggesting that targeting the PERK-histone lactylation axis may be a viable strategy to combat this deadly brain cancer.
