New publications
New horizons in early cancer detection: multicancer tests (MCED) and their prospects
Last reviewed: 02.07.2025

All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.
Cancer remains one of the most serious public health problems, causing significant mortality worldwide. In 2022 alone, there were an estimated 19.3 million new cases of cancer and 10 million cancer-related deaths worldwide. The high mortality rate is primarily due to late detection of the disease, often after it has metastasized, when treatment options are limited. Early detection is key, as it could prevent at least 15% of cancer deaths within five years by allowing precancerous lesions to be removed and localized forms of the disease to be treated.
Cancer is characterized by the uncontrolled proliferation and spread of abnormal cells in the body. While normal cells undergo a regulated process of growth and division, old or damaged cells naturally die and are replaced by new ones. However, when this process is disrupted, it can lead to the formation of tumors, which can be either benign or malignant. Malignant tumors, unlike benign tumors, invade nearby tissues and spread to other parts of the body through metastasis, which is the cause of most cancer-related deaths.
Recent advances in cancer research have led to the development of multi-cancer early detection (MCED) tests. These tests represent a promising approach to detect cancer at its earliest stages by analyzing tumor-related markers in biological fluids such as blood and using artificial intelligence to detect and differentiate between different types of cancer. MCED tests belong to a broader category of liquid biopsies, which are non-invasive and cost-effective alternatives to traditional tissue biopsies. They provide a comprehensive genomic picture of a tumor by detecting specific biological signals in the DNA, RNA, or proteins secreted by cancer cells.
A study on this topic was published in the Journal of Exploratory Research in Pharmacology.
MCED tests offer several advantages, including noninvasiveness, cost-effectiveness, and the ability to perform serial sampling to monitor drug resistance and tumor progression. These tests detect fragments of DNA or RNA released by tumor cells into the bloodstream, helping to identify the most likely origin of the cancer. This capability is key to detecting cancer early, when it is most treatable.
Liquid biopsies, the basis of MCED tests, have revolutionized the approach to cancer detection. Traditional biopsies, which involve surgical removal of tissue, can be invasive, painful, and come with risks of complications. In contrast, liquid biopsies require only a blood sample, making the process significantly less invasive and more acceptable to patients. Not only does this method improve patient comfort, but it also allows for repeated sampling over time, allowing for continuous monitoring of cancer progression or response to treatment.
Additionally, liquid biopsies may better capture tumor heterogeneity than single tissue biopsies because they collect genetic information from cancer cells released into the bloodstream from multiple sites in the body.
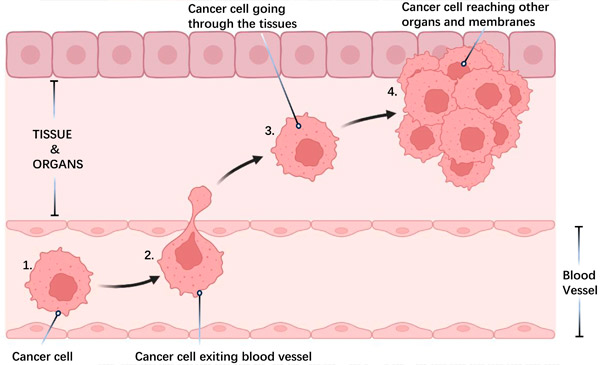
Metastasis of cancer cells:
1) Cell detachment: Cancer cells leave the primary tumor and invade nearby tissues.
2) Vessel entry and travel: Cells enter blood or lymphatic vessels, spreading throughout the body.
3) Tissue attachment: Cells attach to new tissues.
4) Distant tumor formation: New tumors develop at distant sites.
Metastasis, which is the spread of cancer cells from the primary tumor to other organs, is the leading cause of cancer deaths. This process involves various cellular mechanisms, such as infiltration into nearby tissues, evasion of immune system detection and suppression, influence on the local tissue environment, and development of resistance to treatment.
Source: Journal of Exploratory Research in Pharmacology (2024). DOI: 10.14218/JERP.2023.00007
Despite their potential, MCED tests face significant challenges in clinical implementation, including the need for a standardized system to evaluate their efficacy and safety. Currently, only a few MCED tests are available to physicians, and none have been approved for marketing by the Food and Drug Administration (FDA). The specificity of these tests is generally high, but their sensitivity can vary depending on the type and stage of cancer.
The lack of standardized protocols for evaluating MCED assays is a barrier to their widespread adoption. Each assay uses different methodologies, biomarkers, and analytical techniques, making it difficult to compare results across studies or establish universal performance metrics. To address this issue, regulatory agencies and research institutions should collaborate to develop comprehensive guidelines that ensure the reliability and accuracy of MCED assays. This standardization is critical to achieving regulatory approval and integrating these assays into routine clinical practice.
MCED tests can be used both for symptomatic patients to minimize diagnostic delays and for screening apparently healthy individuals to detect asymptomatic cancers. Liquid biopsies, which are the basis of MCED tests, have shown promise in clinical trials, providing a noninvasive means of detecting and monitoring cancer. The US Surveillance, Epidemiology, and End Results program has used state transition models to predict the potential benefits of MCED tests, including diagnostic yield, staging, and mortality reduction.
Several ongoing clinical trials are evaluating the effectiveness of MCED tests. These studies are key to demonstrating the clinical utility of the tests, confirming their ability to detect cancer early and improving patient outcomes. Preliminary results from these trials have shown that MCED tests can detect several types of cancer with high specificity, although sensitivity varies. For example, trials have shown that these tests are particularly effective in detecting cancers that are currently difficult to detect using traditional screening methods, such as pancreatic and ovarian cancer.
The development and implementation of MCED tests represents a significant advance in cancer detection and diagnosis. These tests have the potential to revolutionise cancer screening by detecting multiple cancer types simultaneously at an early stage. However, further research and standardisation are needed to ensure their effectiveness and safety before they become a standard part of clinical practice. Continued innovation and investment in this area is vital to improving cancer survival rates and reducing the global burden of this disease.
