New publications
Major neuron controlling movement in worms discovered, important for human treatment
Last reviewed: 02.07.2025

All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.

Researchers from Sinai Health and the University of Toronto have discovered a mechanism in the nervous system of the tiny roundworm C. elegans that could have significant implications for the treatment of human diseases and the development of robotics.
The study, led by Mei Zhen and colleagues at the Lunenfeld-Tanenbaum Research Institute, is published in the journal Science Advances and reveals the key role of a specific neuron called AVA in controlling the worm's ability to switch between forward and backward movement.
It is essential for worms to crawl toward food sources and retreat quickly from danger. This behavior, where the two actions are mutually exclusive, is typical for many animals, including humans, who cannot sit and run at the same time.
Scientists have long believed that movement control in worms is accomplished through the simple interaction of two neurons: AVA and AVB. The former was thought to promote backward movement, the latter forward movement, with each inhibiting the other to control the direction of movement.
However, new data from Zhen’s team challenges this view, revealing a more complex interaction in which the AVA neuron plays a dual role. Not only does it immediately stop forward movement by suppressing AVB, but it also maintains long-term stimulation of AVB to ensure a smooth transition back to forward movement.
This discovery highlights the ability of the AVA neuron to finely control movement through different mechanisms depending on different signals and on different time scales.
"From an engineering perspective, this is a very economical design," says Zheng, a professor of molecular genetics in the University of Toronto's Temerty School of Medicine. "Strong, sustained inhibition of the feedback loop allows the animal to respond to adverse conditions and escape. At the same time, the control neuron continues to pump a constant gas into the forward loop to move to safe locations."
Jun Meng, a former doctoral student in Zheng's lab who led the study, said understanding how animals transition between such opposing motor states is key to understanding how animals move, as well as for research into neurological disorders.
The discovery of the dominant role of the AVA neuron offers new insights into neural circuitry that scientists have studied since the advent of modern genetics more than half a century ago. Zheng’s lab successfully used cutting-edge technology to precisely modulate the activity of individual neurons and record data from live worms in motion.
Zhen, also a professor of cell and systems biology in the University of Toronto’s Faculty of Arts and Sciences, emphasizes the importance of interdisciplinary collaboration in this study. Meng conducted the key experiments, and electrical recordings from neurons were performed by Bin Yu, a PhD student in Shangbang Gao’s lab at Huazhong University of Science and Technology in China.
Tosif Ahmed, a former postdoctoral fellow in Zheng's lab and now a Theory Fellow at HHMI's Janelia Research Campus in the US, led the mathematical modelling that was important for testing hypotheses and gaining new insights.
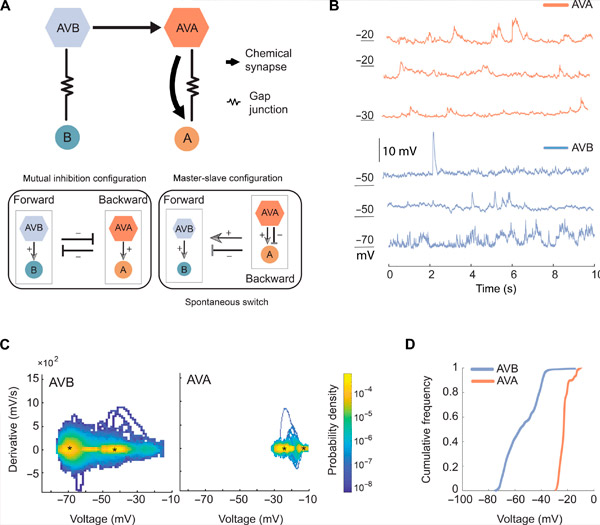
AVA and AVB have different membrane potential ranges and dynamics. Source: Science Advances (2024). DOI: 10.1126/sciadv.adk0002
The study's findings provide a simplified model for studying how neurons can manage multiple roles in movement control - a concept that could also be applied to human neurological conditions.
For example, AVA’s dual role depends on its electrical potential, which is regulated by ion channels on its surface. Zheng is already investigating how similar mechanisms might be involved in a rare condition known as CLIFAHDD syndrome, caused by mutations in similar ion channels. The new findings could also inform the design of more adaptive and efficient robotic systems capable of performing complex movements.
“From the origins of modern science to cutting-edge research today, model organisms like C. elegans have played an important role in uncovering the complexity of our biological systems,” said Anne-Claude Gingras, director of the Lunenfeld-Tanenbaum Research Institute and vice president of research at Sinai Health. “This study is a great example of how we can learn from simple animals and apply that knowledge to advance medicine and technology.”
