Medical expert of the article
New publications
A retina has been grown from human embryonic stem cells
Last reviewed: 01.07.2025

All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.
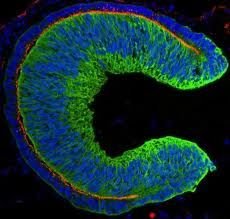
Human stem cells spontaneously form tissue that develops into the retina, the tissue in the eye that allows us to see, according to a paper published in the journal Cell Stem Cell. In the future, transplanting this 3D tissue could help patients with vision problems.
“This is an important milestone in the next stage of regenerative medicine,” said the study’s leader, Professor Yoshiki Sasai, MD, PhD, director of the Organogenesis and Neurogenesis Group, RIKEN Center for Developmental Biology, Japan. “Our approach opens up new perspectives on the use of complex tissues derived from human stem cells for treatment, as well as for medical research related to pathogenesis and drug development.”
During development, the retina – the light-sensitive tissue that lines the inside of the eye – is formed from a structure known as the optic cup. In the new work by Japanese researchers, this structure was spontaneously formed from human embryonic stem cells (hESCs) – cells derived from human embryos that have the potential to differentiate into a variety of tissues. This was made possible by cell culture techniques optimized by Professor Sasai and his team.
HESC-derived cells organize into a regular three-dimensional structure with two layers of the optic cup, one of which contains a large number of light-sensitive cells called photoreceptors. Since retinal degeneration primarily results from damage to photoreceptors, hESC-derived tissue may be an ideal transplant material.
The research by Japanese scientists not only opens up further prospects for the use of stem cells in regenerative medicine, but will certainly accelerate the development of such a field of natural science as developmental biology. During the experiments, the researchers became convinced that the optic cup formed from human embryonic stem cells is much thicker than that grown from mouse embryonic stem cells. In addition, it contains both rods and cones, while differentiation into cones is rarely observed in the culture of mouse ESCs. This means that the embryonic cells carry species-specific instructions for creating this eye structure.
"Our study opens the way to understanding the developmental features of the eye that are specific to humans and that have previously been impossible to study," says Professor Sasai.

This is not the first major success of Professor Sasai's group. Late last year, the scientists grew a functional anterior pituitary gland (adenohypophysis) from mouse embryonic stem cells, consisting of several different types of hormone-producing cells. An article about the results of this work, Self-formation of functional adenohypophysis in three-dimensional culture, was published in the journal Nature.
The pituitary gland is a small endocrine gland at the base of the brain that produces several important hormones. It is particularly important during early development, and being able to mimic its formation in the lab will help scientists better understand embryogenesis. Abnormalities in the pituitary gland have been associated with growth disorders such as gigantism and vision problems including blindness.
This experiment would not have been possible without 3D cell culture. The pituitary gland is a separate organ, but its development requires chemical signals from the area of the brain directly above it, the hypothalamus. In 3D culture, the scientists were able to grow two types of tissue side by side at the same time, resulting in stem cells that self-organized into the pituitary gland after two weeks.
Fluorescent staining showed that the cultured pituitary tissue expressed the appropriate biomarkers and secreted hormones typical of the anterior pituitary gland. The researchers went one step further and tested the functionality of the organs they had synthesized by transplanting them into mice lacking a pituitary gland. The experiments were successful: the bioengineered pituitaries restored the levels of glucocorticoid hormones in the animals’ blood and eliminated behavioral symptoms such as lethargy. The condition of the mice with implanted structures made of stem cells, which were not exposed to the necessary signaling factors and therefore did not become a functional pituitary gland, did not improve.
Professor Sasai and his colleagues plan to repeat the experiment on human stem cells and they believe this work will take at least three years.


 [
[