New publications
Activation of innate immunity: an important part of the mechanism identified
Last reviewed: 02.07.2025

All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.
Researchers from LMU have deciphered the complex interaction of various enzymes around the innate immune receptor Toll-like receptor 7 (TLR7), which plays an important role in protecting our body from viruses.
Toll-like receptor 7 (TLR7), located on the dendritic cells of our immune system, plays a critical role in our natural defense against viruses. TLR7 recognizes single-stranded viral and other foreign RNA and activates the release of inflammatory mediators. Dysfunctions of this receptor also play a key role in autoimmune diseases, making understanding and, ideally, modulating the mechanism of TLR7 activation even more important.
The researchers, led by Professor Veit Hornung and Marlene Berouti from the Genetics Centre Munich and the Department of Biochemistry at LMU, were able to delve deeper into the complex activation mechanism. It was known from previous studies that complex RNA molecules must be cut so that the receptor can recognise them.
Using a range of technologies from cell biology to cryo-electron microscopy, LMU researchers have uncovered how single-stranded foreign RNA is processed to detect TLR7. Their work was published in the journal Immunity.
Numerous enzymes are involved in the recognition of foreign RNA
Over the course of evolution, the immune system has specialized in recognizing pathogens by their genetic material. For example, the innate immune receptor TLR7 is stimulated by viral RNA. We can think of viral RNA as long strands of molecules that are too large to be recognized as ligands for TLR7. This is where nucleases come in — molecular cutting tools that cut the “RNA strand” into small pieces.
Endonucleases cut the RNA molecules down the middle, like scissors, while exonucleases cleave the strand from one end to the other. This process generates different RNA fragments that can now bind to two different pockets on the TLR7 receptor. Only when both binding pockets on the receptor are occupied by these RNA pieces is a signaling cascade triggered that activates the cell and triggers an alarm state.
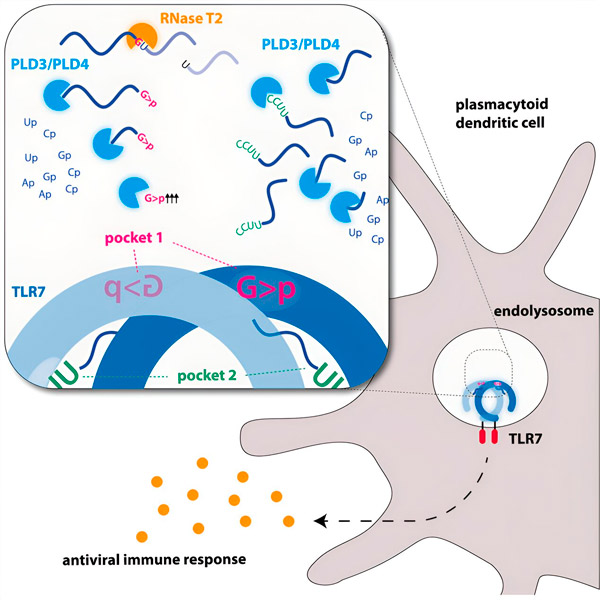
Graphic image. Source: Immunity (2024). DOI: 10.1016/j.immuni.2024.04.010
The researchers found that TLR7 RNA recognition requires the activity of the endonuclease RNase T2, acting in conjunction with the exonucleases PLD3 and PLD4 (phospholipase D3 and D4). "While it was known that these enzymes could degrade RNA," says Hornung, "we have now demonstrated that they interact with and thereby activate TLR7."
Balancing the immune system
The researchers also found that PLD exonucleases play a dual role in immune cells. In the case of TLR7, they have a pro-inflammatory effect, while in the case of another TLR receptor, TLR9, they have an anti-inflammatory effect. “This dual role of PLD exonucleases indicates a finely coordinated balance for controlling proper immune responses,” explains Berouti.
"The simultaneous stimulation and inhibition of inflammation by these enzymes may serve as an important protective mechanism to prevent dysfunctions in the system." What role other enzymes may play in this signaling pathway and whether the molecules involved are suitable as target structures for therapy will be the subject of further research.
