Lymphocytes mobilize the immune system against aggressive breast cancer
Last reviewed: 14.06.2024

All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.
Researchers from the Autonomous University of Barcelona (UAB) and the Hospital del Mar Research Institute have confirmed that patients who have NK lymphocytes around their tumors show a better response to treatment. This supports the use of cytokines released by NK cells as markers of treatment response using a simple blood test and supports the use of these lymphocytes to enhance treatment in patients with metastatic HER2-positivebreast cancer .
NK cells, known for their anti-tumor properties, can, when combined with treatment against the most aggressive form of breast cancer, activate the immune system to detect cancer cells. This ability allows them to attract other cells of the immune system to fight the tumor.
Discovery of a potential biomarker
Research published in the Journal of Experimental & Clinical Cancer Researchalso allowed researchers to describe a potential biomarker for identifying patients who respond to treatment.
The study was led by scientists from the Immune and Infection Research Group at the Hospital del Mar Research Institute, Dr. Aura Muntasell, who also teaches at UAB, and graduate student Sarah Santana.
Previous research and new discoveries
Previous research from this group has confirmed that the presence of NK cells, a type of cytotoxic lymphocyte that can kill tumor cells, in HER2-positive breast cancer tumors is associated with patient response to anti-HER2 antibody treatment. However, despite this association, their numbers were lower than those of other immune system cells, so researchers suspected they also played a regulatory role in the body's response to cancer.
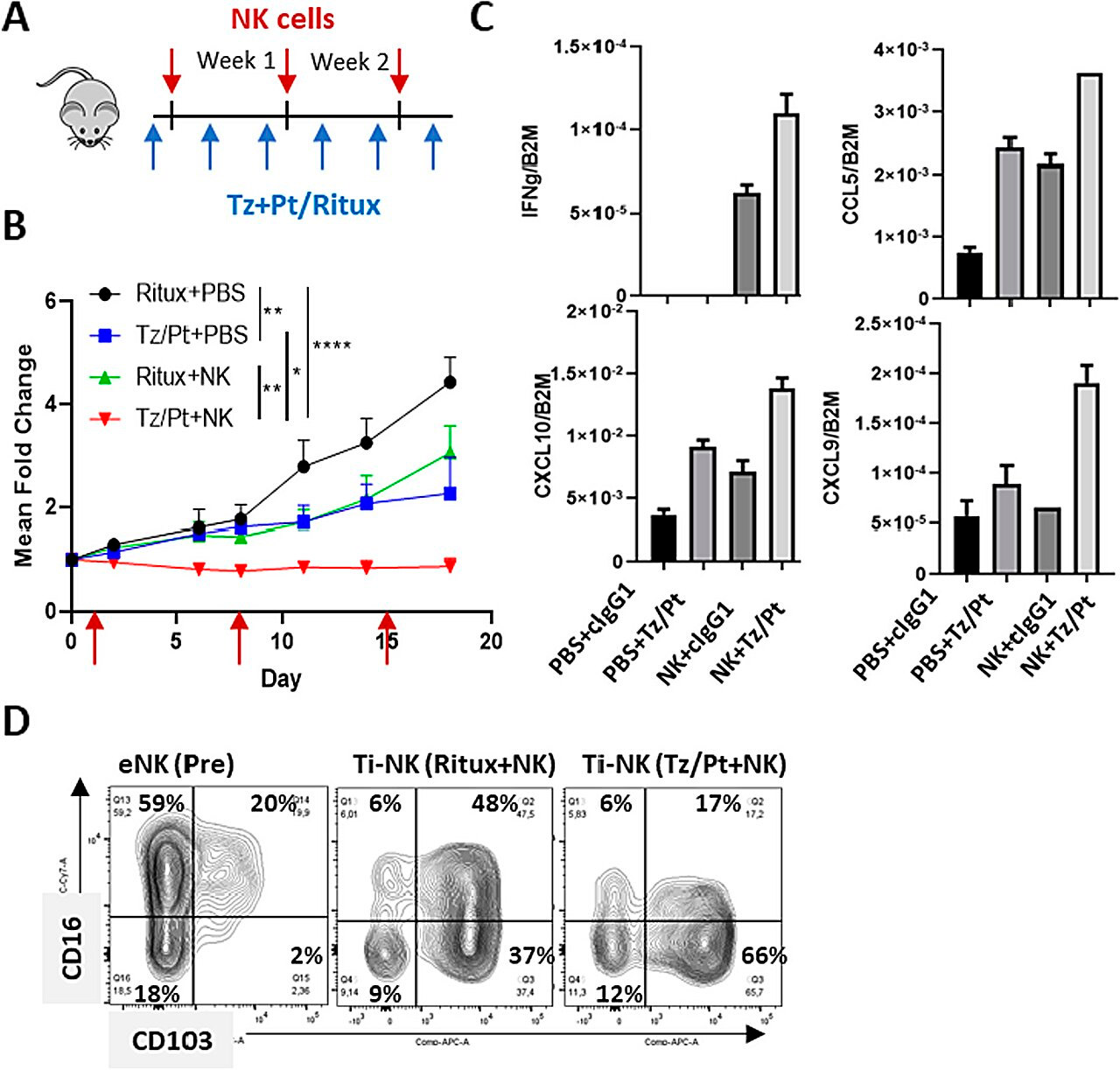
Treatment with a combination of NK cells and anti-HER2 antibodies in a humanized mouse model of HER2-positive breast cancer. Source: Journal of Experimental & Clinical Cancer Research (2024). DOI: 10.1186/s13046-023-02918-4
New research has focused on clarifying this aspect. By comparing sets of RNA biopsies from HER2-positive breast cancer tumors with and without NK cells, as well as mouse models, the work was able to demonstrate that these cells, when exposed to antibodies used against these tumors, release two types of small proteins—cytokines and other soluble factors.
This changes the tumor microenvironment, which in turn makes it easier for other immune system cells to enter, enhancing the effect of cancer treatment.
Potential new biomarker of treatment response
The study also examined whether patients could detect, through blood or serum testing, factors released by NK cells when exposed to anti-HER2 antibody treatment. Through serum samples from patients during treatment, their presence was confirmed in cases where the person had a positive response.
"New evidence confirms the ability of anti-HER2 therapies to induce an immune response that correlates with greater therapeutic efficacy. This should serve as the basis for further improvement and personalization of treatment for patients with HER2 positive breast cancer," said Dr. Joan Albanel, Chief Executive Officer of the Oncology Department of the Hospital del Mar, Director of the Cancer Research Program of the Institute for Research of the Hospital del Mar and one of the authors of the study.
Translation of findings to other types of tumors
The researchers believe these findings may be transferable to other types of tumors, as the study "provides evidence that the activity of NK lymphocytes as cells capable of altering the tumor environment can be translated to other tumors," Dr. Muntasell explained.
