Closed-loop drug delivery system could improve chemotherapy
Last reviewed: 14.06.2024

All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.
When cancer patients undergo chemotherapy, doses of most drugs are calculated based on the patient's body surface area. This indicator is estimated using an equation into which the patient's height and weight are substituted. This equation was formulated in 1916 based on data from just nine patients.
This simplistic approach to dosing does not take into account other factors and may result in the patient being prescribed too much or too little of the drug. As a result, some patients may experience excessive toxicity or lack of effectiveness from their chemotherapy.
To improve the accuracy of chemotherapy dosing, MIT engineers have developed an alternative approach that allows the dose to be personalized for each patient. Their system measures the amount of drug in the patient's body, and this data is entered into the controller, which can adjust the infusion rate accordingly.
This approach may help compensate for differences in drug pharmacokinetics caused by body composition, genetic predisposition, chemotherapy-induced organ toxicity, interactions with other medications and foods, and circadian fluctuations in enzymes responsible for breaking down chemotherapy drugs, the researchers say. p>
"By recognizing advances in understanding how drugs are metabolized and applying engineering tools to simplify personalized dosing, we believe we can help transform the safety and effectiveness of many drugs," says Giovanni Traverso, an assistant professor of mechanical engineering at MIT and a gastroenterologist at the hospital. Brigham and Women's Hospital and senior author of the study.
Louis DeRidder, an MIT graduate student, is the lead author of the paper published in Med.
Continuous monitoring
In this study, the researchers focused on a drug called 5-fluorouracil, which is used to treat colorectal cancer and other types of cancer. The drug is usually administered over a 46-hour period and dosage is determined using a formula based on the patient's height and weight, which provides an estimate of body surface area.
However, this approach does not account for differences in body composition, which may affect the distribution of the drug in the body, or genetic variations that affect its metabolism. These differences can lead to harmful side effects if there is too much of the drug. If the drug is not enough, it may not kill the tumor as expected.
"People with the same body surface area may have very different heights and weights, different muscle mass or genetics, but as long as the height and weight put into this equation give the same body surface area, their dose is identical," says DeRidder, a PhD candidate in the medical engineering and medical physics program at the Harvard-MIT Health Sciences and Technology Program.
Another factor that can change the amount of drug in the blood at any given time is the circadian fluctuation of an enzyme called dihydropyrimidine dehydrogenase (DPD), which breaks down 5-fluorouracil. The expression of DPD, like many other enzymes in the body, is regulated by a circadian rhythm. Thus, the degradation of 5-FU DPD is not constant, but varies depending on the time of day. These circadian rhythms can result in tenfold fluctuations in the amount of 5-fluorouracil in a patient's blood over the course of an infusion.
"Using body surface area to calculate chemotherapy dose, we know that two people can have completely different toxicities from 5-fluorouracil. One patient can have treatment cycles with minimal toxicity and then a cycle with terrible toxicity. Something changed in how this patient metabolized chemotherapy from one cycle to the next. Our outdated dosing method does not capture these changes, and patients suffer as a result,” says Douglas Rubinson, clinical oncologist at Dana-Farber Cancer Institute and author of the paper.
One way to try to compensate for variability in chemotherapy pharmacokinetics is a strategy called therapeutic drug monitoring, in which the patient provides a blood sample at the end of one treatment cycle. After this sample is analyzed for drug concentrations, the dosage can be adjusted, if necessary, at the start of the next cycle (usually after two weeks for 5-fluorouracil).
This approach has been shown to lead to better outcomes for patients, but has not been widely used for chemotherapies such as 5-fluorouracil.
MIT researchers wanted to develop a similar type of monitoring, but in an automated way that could personalize drug dosage in real time, which could lead to better outcomes for patients.
In their closed-loop system, drug concentrations can be continuously monitored and this information is used to automatically adjust the chemotherapy drug infusion rate to maintain the dose within the target range.
This closed-loop system allows drug dosing to be personalized to take into account circadian rhythms of changing levels of drug-metabolizing enzymes, as well as any changes in the patient's pharmacokinetics since last treatment, such as chemotherapy-induced organ toxicity.
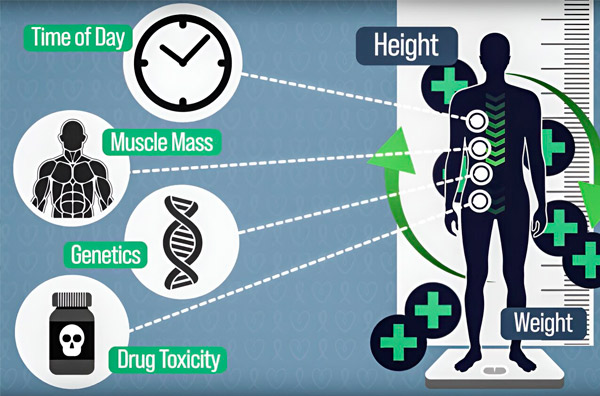
To make chemotherapy dosing more precise, MIT engineers have developed a way to continuously measure the amount of drug in a patient's body during an hours-long infusion. This will help compensate for differences caused by body composition, genetics, drug toxicity, and circadian fluctuations. Source: Provided by researchers.
The new system developed by the researchers, known as CLAUDIA (Closed-Loop AUtomated Drug Infusion regulAtor), uses commercially available equipment for each step. Blood samples are taken every five minutes and quickly prepared for analysis. The concentration of 5-fluorouracil in the blood is measured and compared with the target range.
The difference between the target and measured concentrations is entered into the control algorithm, which then adjusts the infusion rate if necessary to maintain the dose within the concentration range at which the drug is effective and non-toxic.
“We have developed a system in which we can continuously measure drug concentrations and adjust the infusion rate accordingly to maintain drug concentrations within the therapeutic window,” says DeRidder.
Quick adjustment
In animal tests, the researchers found that using CLAUDIA, they were able to keep the amount of drug circulating in the body in the target range about 45 percent of the time.
Drug levels in animals receiving chemotherapy without CLAUDIA remained in the target range only 13 percent of the time on average. In this study, the researchers did not test the effectiveness of drug levels, but maintaining concentrations within the target window is believed to result in better results and less toxicity.
CLAUDIA was also able to maintain the dose of 5-fluorouracil in the target range even when administered a drug that inhibits the DPD enzyme. In animals treated with this inhibitor without continuous monitoring and adjustment, 5-fluorouracil levels increased up to eightfold.
For this demonstration, the researchers manually performed each step of the process using off-the-shelf equipment, but now plan to automate each step so that monitoring and dose adjustments can be done without human intervention.
To measure drug concentrations, the researchers used high-performance liquid chromatography-mass spectrometry (HPLC-MS), a technique that can be adapted to detect virtually any type of drug.
“We envision a future in which we can use CLAUDIA for any drug that has suitable pharmacokinetic properties and is detectable by HPLC-MS, allowing personalized dosing for many different drugs,” says DeRidder.
