New publications
FDA-approved TIVDAK®: Targeting tissue factor in cervical cancer
Last reviewed: 02.07.2025

All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.
On April 29, 2024, the U.S. Food and Drug Administration (FDA) granted full approval to Seagen Inc.’s TIVDAK® (tisotumab vedotin), a tissue factor (TF)-targeted drug, for the treatment of patients with recurrent or metastatic cervical cancer that has progressed during or after chemotherapy. This represents a significant breakthrough in cervical cancer therapy, highlighting the potential of antibody drug conjugates (ADCs) in oncology.
Mechanism of action of TIVDAK
Tivdak is an ADC that targets TF by combining Genmab's anti-TF monoclonal antibody tisotumab with Seagen's ADC technology designed to target TF antigens on cancer cells and deliver the cytotoxic component MMAE directly to cancer cells.
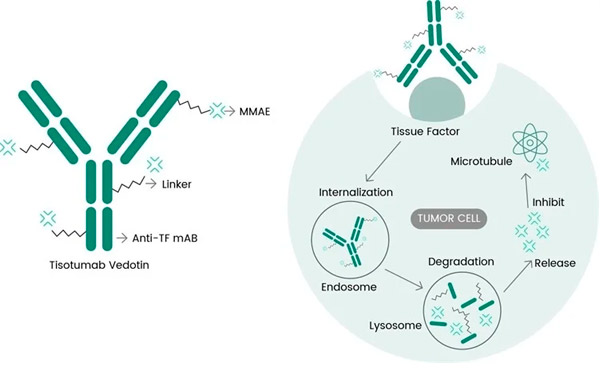
Molecular mechanism of action of tisotumab vedotin ( https://doi.org/10.3390/ijms23073559 )
TF: The Perfect Target for ADC Development
TF is known to be involved in tumor signaling and angiogenesis and is overexpressed in the vast majority of patients with cervical cancer and many other solid tumors. Its ability to be rapidly internalized upon antibody binding and its minimal impact on normal blood coagulation further enhances its suitability for targeted cancer therapy.
