New publications
Thalidomide derivative compounds lead to the death of resistant cancer cells
Last reviewed: 02.07.2025

All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.
A study conducted by Goethe University in Frankfurt points to the possibility that thalidomide derivatives could potentially be used to treat cancer. Thalidomide was sold as a sleeping pill in the 1950s. It later became notorious for causing severe birth defects in the early stages of pregnancy.
The molecule is also known to mark proteins in the cell for destruction. As part of the current study, scientists created derivatives of thalidomide. They were able to show that these substances affect the destruction of proteins responsible for the survival of cancer cells.
Perhaps no other molecule has had such a turbulent past as thalidomide. It was the main ingredient in a drug approved in many countries in the 1950s as a sedative and sleeping pill. But it soon became clear that pregnant women taking thalidomide often gave birth to babies with serious deformities.
However, in recent decades, medicine has once again placed great hopes on it. Studies have shown, among other things, that it inhibits the growth of blood vessels and is therefore potentially suitable for cutting off tumors from their nutrient medium. Then it also proved very effective in the treatment of multiple myeloma, malignant tumors in the bone marrow.
"We now know that thalidomide can be called a 'molecular glue'," explains Dr Xinglai Cheng of the Institute of Pharmaceutical Chemistry at Goethe University Frankfurt. "This means that it is able to grab two proteins and join them together."
This is particularly interesting because one of these proteins is a kind of "labeling machine": it attaches an unambiguous "GARBAGE" label to another protein.
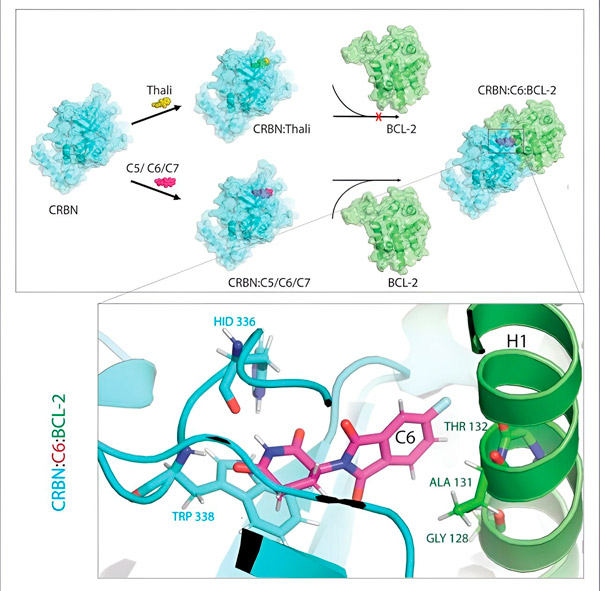
Thalidomide derivatives C5, C6, and C7 alter CRBN, the "labeling machine," so that it can bind to BCL-2. In this way, the BCL-2 molecule is marked for degradation—a possible new strategy to fight cancer. Author: Dr. Xinglai Cheng.
The cell's waste disposal system recognizes this tag: It grabs the tagged protein molecule and shreds it. "This mechanism explains the different effects of thalidomide," says Cheng. "Depending on which protein is tagged, it can cause deformities during embryonic development or destroy malignant cells."
This mechanism opens up great possibilities for medicine, since cancer cells depend on certain proteins to survive. If they could be systematically targeted and shredded, perhaps the disease could be cured. The problem is that the molecular glue is quite peculiar.
One of its binding partners is always the cell's labeling machine, or, in scientific parlance, an E3 ligase called CRBN. Only a very few of the many thousands of proteins in the body can be the second partner—which ones depends on the glue.
"So we created a series of thalidomide derivatives," says Cheng. "We then tested whether they had adhesive properties and, if so, which proteins they were effective against." To do this, the researchers added their derivatives to all the proteins in the cultured cell line. They then observed which of these proteins were then degraded in the presence of CRBN.
"In the process, we identified three derivatives that could tag a cellular protein that is very important for degradation, BCL-2," explains Cheng. "BCL-2 prevents the cells from activating their self-destruction program, so if it's not there, the cells die."
That's why BCL-2 has long been a focus of cancer research. There's even a leukemia drug called venetoclax that reduces the effectiveness of BCL-2 and thus causes mutated cells to self-destruct.
"However, in many cancer cells, BCL-2 itself is mutated. As a result, venetoclax no longer inhibits the protein," says Cheng. "We were able to show that our derivatives also flag this mutated form for degradation. In addition, our partners at the Max Planck Institute for Biophysics simulated the interaction of thalidomide derivatives with BCL-2 on a computer. This showed that the derivatives bind to completely different sites than venetoclax - a result that we were later able to confirm experimentally."
The researchers also tested their compounds on fruit flies with cancer cells. The survival rate of the flies treated in this way was significantly higher. However, Cheng cautions against getting your hopes up too much, as these results are still basic research. "While they show that the modified thalidomide molecules have great therapeutic potential, we cannot yet say whether they will prove themselves in practice at some point in time."
The results of the study were published in the journal Cell Reports Physical Science.
