New publications
Scientists uncover molecular mechanisms of donor arteries for coronary artery bypass grafting
Last reviewed: 02.07.2025

All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.

Coronary artery bypass grafting (CABG) is a surgical procedure that improves blood flow to the heart tissue and effectively treats myocardial ischemia caused by coronary artery disease. In CABG, a healthy blood vessel is taken from the patient and connected to the diseased artery so that blood can bypass the blocked section of the coronary artery.
The main healthy vessels used for CABG include the internal mammary artery (ITA), radial artery (RA), and right gastroepiploic artery (RGA). Among these donor arteries, ITA provides the best long-term outcome, while RA and RGA are susceptible to intimal hyperplasia, atherosclerosis, and vasospasm.
A team led by Wang Xiujie from the Institute of Genetics and Developmental Biology (IGDB) of the Chinese Academy of Sciences, in collaboration with a team led by Song Jianping from Fuwai Hospital of the Chinese Academy of Medical Sciences, used single-nucleus RNA sequencing (scRNA-seq) to study the cell type composition and genetic expression profiles of ITA, RA, and RGA.
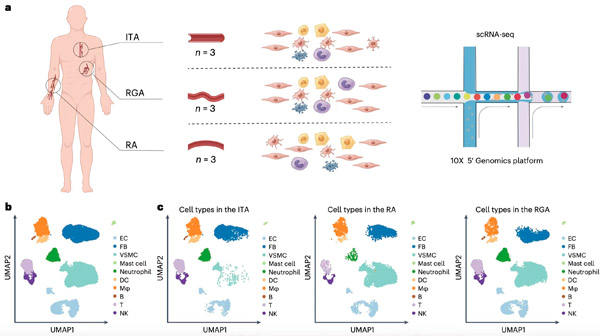
Profiling of 38,814 single cells isolated from three types of donor arteries. a. Schematic of the overall study design; b. UMAP plot of the pooled donor artery datasets, cells colored according to major cell types; c. UMAP plots showing the composition and similarities of the major cell types in each donor artery. Source: IGDB
The researchers found that these three types of donor arteries differ in their ability to absorb lipid particles, hemodynamics, vasospasm, and fibrosis. Combined with experimental validation in human cells and mice, the following four optimized strategies for CABG were proposed: inhibition of macrophage migration factor can reduce intimal hyperplasia of RA; potassium channel activators can counteract vasospasm of RGA unresponsive to calcium antagonists; inhibition of CREB5 and GDF10 can reduce extracellular matrix deposition and fibrosis in RA and RGA; PCSK9 inhibitors are recommended for lipid-lowering treatment in ITA.
This study is expected to provide guidance for the development of clinical strategies for CABG and the choice of postoperative medications.
The paper, titled "Strategies for optimizing arterial grafts at the single-cell level," was published in the journal Nature Cardiovascular Research.
