New publications
Scientists have created a 'chameleon' compound to treat drug-resistant brain cancers
Last reviewed: 02.07.2025

All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.
A new study by Yale University scientists describes how a new chemical compound attacks drug-resistant brain tumors without damaging healthy surrounding tissue.
The study, published in the Journal of the American Chemical Society, is an important step in the development of so-called "chameleon compounds" that could be used to fight a range of dangerous cancers.
Gliomas develop in approximately 6.6 per 100,000 people each year and in 2.94 per 100,000 people by age 14. Excluding metastases from other cancers that reach the central nervous system, gliomas account for 26% of all brain tumors (primary brain tumors) and 81% of all malignant brain tumors.
For decades, patients with glioblastoma have been treated with a drug called temozolomide. However, most patients develop resistance to temozolomide within a year. The five-year survival rate for patients with glioblastoma is less than 5%.
In 2022, Yale chemist Seth Herzon and radiation oncologist Dr. Ranjit Bindra developed a new strategy to treat glioblastomas more effectively. They created a class of anti-cancer molecules called chameleon compounds that exploit a defect in a DNA repair protein known as O6-methylguanine DNA methyltransferase (MGMT).
Many cancer cells, including glioblastomas, lack the MGMT protein. New chameleon compounds are designed to damage DNA in tumor cells lacking MGMT.
Chameleon compounds initiate DNA damage by depositing primary lesions on DNA that over time evolve into highly toxic secondary lesions known as interstrand crosslinks. MGMT protects the DNA of healthy tissues by repairing primary lesions before they can evolve into deadly interstrand crosslinks.
For their new study, co-authors Herzon and Bindra focused on their lead chameleon, KL-50.
"We used a combination of synthetic chemistry and molecular biology studies to elucidate the molecular basis of our previous observations, as well as the chemical kinetics that provide the unique selectivity of these compounds," said Herzon, the Milton Harris Professor of Chemistry at Yale. "We show that KL-50 is unique in that it forms DNA cross-links only in tumors with defective DNA repair. It spares healthy tissue."
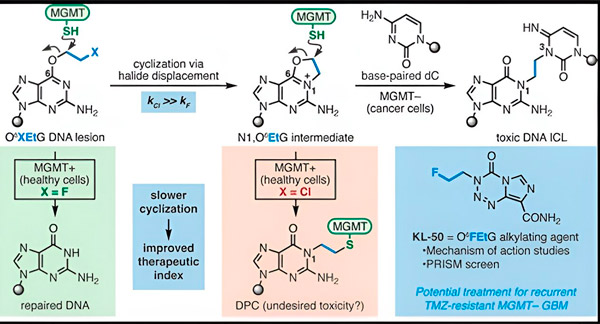
Source: Journal of the American Chemical Society (2024). DOI: 10.1021/jacs.3c06483
That's a significant difference, the researchers point out. A number of other anti-cancer compounds have been designed to trigger interstrand cross-links, but they are not selective for tumor cells, limiting their usefulness.
The secret to KL-50’s success is its timing, the researchers noted. KL-50 forms interstrand crosslinks more slowly than other crosslinkers. This delay gives healthy cells enough time to use MGMT to prevent crosslinks from forming.
"This unique profile suggests its potential for the treatment of drug-resistant glioblastoma, an area with a large unmet need in the clinic," said Bindra, the Harvey and Kate Cushing Professor of Therapeutic Radiology at Yale School of Medicine. Bindra is also scientific director of the Chenevert Family Brain Tumor Center at Smilo Hospital.
Herzon and Bindra said their study highlights the importance of considering the rates of chemical DNA modification and biochemical DNA repair. They believe they can use this strategy to develop treatments for other cancers that contain specific tumor-associated DNA repair defects.
