New publications
Researchers identify mutations that protect against B-cell cancer
Last reviewed: 02.07.2025

All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.
Researchers at the University of Texas Southwestern Medical Center were able to suppress leukemia and lymphoma in a mouse model genetically predisposed to these cancers by completely or partially depleting a protein called midnolin in B cells.
Their findings, published in the Journal of Experimental Medicine, could lead to new treatments for these diseases that avoid the serious side effects of current therapies.
"We used a purely genetic approach to find a drug target, and this target was sensational because B-cell leukemias and lymphomas are highly dependent on it, while most host tissues are not," said study leader Bruce Beutler, MD, director of the Center for Genetic Host Defense and professor of immunology and internal medicine at the University of Texas Southwestern Medical Center.
Dr. Beutler, who won the Nobel Prize in Physiology or Medicine in 2011 for discovering an important group of pathogen sensors known as Toll-like receptors found on immune cells, has long used mutagenesis — introducing mutations into the genes of animal models by exposure to a chemical called N-ethyl-N-nitrosourea (ENU) — as a key tool for studying gene function.
Recently, Beutler's lab developed a method known as automated meiotic mapping (AMM), which traces unusual features in mutant mice back to causative mutations, thereby identifying genes needed to maintain normal physiological state.
Mutagenesis often causes genetic diseases in animals, providing insight into the function of the affected genes by studying abnormalities in the animals. However, as Dr. Beutler explained, mutations can also provide protection against disease.
Examples include mutations that protect people with HIV or hereditary sickle cell disease from developing symptoms. The mechanisms underlying some protective mutations have inspired the development of drugs to treat a variety of diseases.
In search of protective mutations for immune disorders, the researchers screened mutant mice for immune cells with unusual features. In several sets of animals with unusually low numbers of B cells — an important component of the adaptive immune system responsible for producing antibodies — the researchers used AMM to trace the deficiency to mutations in midnolin, a protein found primarily in B cells.
Although animals completely lacking midnolin die during development before birth, milder mutations, including some introduced through genetic techniques that allow the gene to be deleted in adulthood, cause no apparent harm.
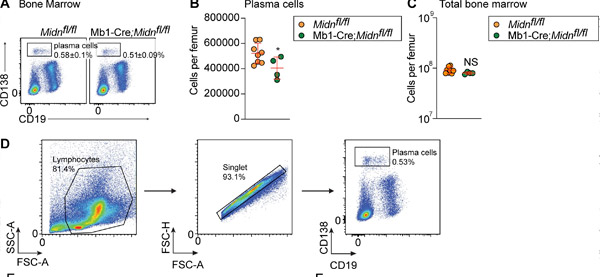
Plasma cell production after immunization with TD antigen β-galactosidase in Mb1-Cre;Midn fl/fl mice. (A and B) Representative flow cytometry graphs (A) and numbers (B) of plasma cells in the bone marrow of 8-week-old Mb1-Cre;Midn fl/fl and Midn fl/fl mice after immunization with β-galactosidase. (C) Total bone marrow cells per femur. (D) Plasma cell isolation strategy. Source: Journal of Experimental Medicine (2024). DOI: 10.1084/jem.20232132
The researchers significantly reduced or eliminated midnolin in mice genetically predisposed to B-cell leukemia and lymphoma, cancers in which B cells divide uncontrollably. Although mice with normal levels of midnolin died from these diseases by 5 months, most of those with less or no midnolin never developed malignant tumors.
Additional experiments showed that midnolin’s role in B cells is to stimulate the activity of proteasomes, cellular organelles that dispose of damaged or no longer needed proteins. Some therapies currently used to treat B-cell leukemia and lymphoma work by inhibiting proteasome activity, much like removing midnolin does, Dr. Beutler explained.
However, unlike these drugs, which have many potentially serious side effects, eliminating or reducing midnoline in animal models did not appear to have any negative effects.
Future research will focus on developing drugs that inhibit midnolin, which may ultimately form the basis for new treatments for B-cell cancers.
