New publications
Parathyroid hormone treatment helps slow the development of osteoarthritis
Last reviewed: 02.07.2025

All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.

Cornell researchers have found that pre-treatment with parathyroid hormone, commonly used to increase bone mass in osteoporosis, may help improve cartilage health and slow the progression of osteoarthritis.
The team, led by Marjolijn van der Meulen, director of the James M. and Marsha McCormick School of Biomedical Engineering at Cornell's College of Engineering, also identified gene expression signatures that could potentially be used for early detection of degenerative joint disease.
The results were published in the journal Science Advances. The article's co-authors are Adrien Antoinette and Sofia Zimyan.
Van der Meulen specializes in studying the role of mechanics in the skeleton and how the musculoskeletal system – bones, cartilage, joints – responds to loading using weight-bearing and compression techniques on the lower leg and knee joint.
Loading has its pros and cons. It increases bone mass and can be used as a therapy for osteoporosis. At the same time, loading also damages the cartilage in joints, similar to the degeneration seen in osteoarthritis. Van der Meulen and her lab are increasingly focused on the role that bone plays in the development of joint damage.
In the new study, the team conducted a two-step process. First, they treated mice daily with parathyroid hormone, a drug prescribed for osteoporosis, to increase bone mass for eight weeks. In the second phase, the team applied daily weight-bearing stress to the mice’s tibias and used another osteoporosis drug, alendronate, which effectively turns off bone’s ability to repair itself (remodel) for six weeks.
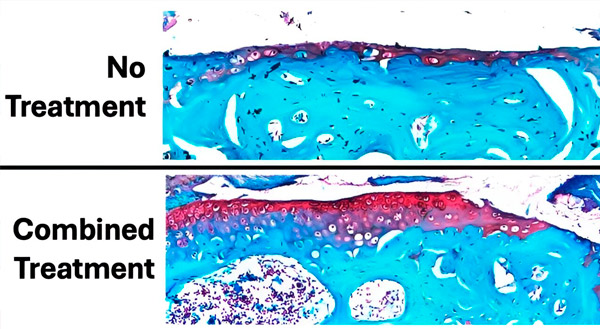
The figure shows the extent of cartilage damage after 6 weeks of daily loading and treatment compared to a control knee with no loading and no cartilage damage. The cartilage is stained red and the bone is bluish-green. Overall, pre-treatment with parathyroid hormone before loading and treatment with alendronate during loading showed the least cartilage damage (loss of red-stained tissue) and the best preservation of cartilage. Source: Science Advances (2024). DOI: 10.1126/sciadv.adk8402
The researchers found that parathyroid hormone directly improved cartilage health and slowed the progression of damage, while alendronate reduced subchondral bone changes associated with osteoarthritis.
"Even after six weeks of damage, the effect of the eight-week pretreatment was still significant. PTH did more than just increase bone mass, because it turns out it also affects cartilage," van der Meulen said. "The mice's knees had thicker cartilage after eight weeks, which was unexpected. Thicker cartilage probably protects against further joint damage."
The team repeated the experiment and used transcriptomics to analyze gene expression in RNA extracted from cartilage, bone, and lymph nodes of mice. Joint damage was reflected in early transcriptomic changes, and both treatments together led to early modulation of immune signaling.
"Gene expression showed that both drugs together had the greatest effect in reducing the expression of genes associated with cartilage damage, particularly altering the expression of immune genes," Zimyan said.
The next step is to determine whether treatment with parathyroid hormone can slow or even reverse the progression of osteoarthritis once it has occurred and to use gene signatures to develop early diagnostics for the disease.
"These results suggest that these treatments may be useful in humans as well. And the good news is that these treatments are already FDA approved, although not for this use," van der Meulen said.
