New publications
Neuroprosthesis for the gastrointestinal tract: restores peristalsis and turns on "satiety hormones"
Last reviewed: 18.08.2025

All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.
Gastrointestinal (esophagus and stomach) motility disorders—achalasia, gastroparesis, dysphagia, etc.—affect more than 20% of the population and cause significant morbidity and costs. Standard approaches—medications, behavioral interventions, and surgery—often have limited effectiveness and do not restore coordinated peristalsis.
- Why existing devices don’t solve the problem. Electrical stimulation of the gastrointestinal tract has been studied since the 1960s, but clinically approved implants (e.g., Enterra for gastroparesis, VBLOC vagal stimulators for obesity, InterStim sacral stimulation for fecal incontinence) operate primarily in an open loop and often produce inconsistent effects on gastric emptying. The reason is that one or more current sources with constant parameters do not reproduce the spatiotemporal complexity of natural peristalsis.
- Physiology that must be “imitated.” Peristalsis is a closed loop: sensory signals (stretch, temperature, chemical stimuli) → reflex responses in the myenteric plexus and smooth muscles. In addition to food transport, motility affects afferent gut-brain signals and satiety hormones (GLP-1, insulin, ghrelin), forming appetite and a feeling of satiety. In dysmotility, these loops are disrupted.
- Technological gap. To reproduce the “correct” waves, multichannel stimulation is needed directly near the myenteric plexus and muscular layer. But access there usually requires invasive surgery; advanced endoscopic techniques (e.g. NOTES) are complex and not widely used. Minimally invasive instruments are needed that allow precise placement of electrodes in the submucosa and work in a closed “sensing → stimulation” loop.
- What the new work offers. The authors describe an endoscopically installed, multichannel neuroprosthesis with electrical and chemical stimulation, capable of triggering coordinated peristaltic waves upon a signal about the passage of a bolus, thereby not only restoring motility, but also modulating the metabolic response (bringing it closer to a “fed” state). This closes key gaps: access to the desired layer, spatiotemporal coordination, and work in a closed loop.
In short: there is a large clinical niche - widespread, poorly treated dismotivations. Previous "open" stimulants do not imitate natural physiology. Therefore, it is logical to try to teach the implant to "think like the gastrointestinal tract": to sense the bolus and trigger physiologic peristalsis exactly where the natural signal passes - at the myenteric plexus.
A team from MIT, Harvard, and Brigham created a miniature esophageal/stomach implant that senses a food bolus in a “closed loop” and triggers coordinated waves of peristalsis. In pigs, the device not only restored esophageal and gastric motility, but also induced hormonal changes similar to the postprandial (fed) state. The implant is placed endoscopically, without abdominal surgery. The study was published in the journal Nature.
What did they come up with?
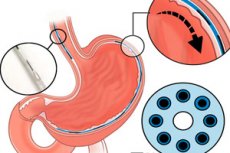
- The implant itself. A thin “fibrous” neuroprosthesis with a diameter of ≈1.25 mm with seven electrodes every 1 cm and a microchannel for local delivery of substances (electro- and chemostimulation). Its flexibility and dimensions allow it to be inserted through a standard instrument channel of an endoscope (2.8–3.2 mm).
- Installation. An endoscopic instrument has been developed: a needle with a reverse pull of a nitinol "hook", hydrodissection, and the key trick - searching for the submucosa by tissue impedance for precise placement just above the muscular layer, near the myenteric plexus.
- Closed loop. The system reads the bolus signal (EMG/intraluminal sensors) and selects a stimulation pattern to induce sequential contractions similar to natural peristalsis. It is possible to combine “excitatory” and “inhibitory” stimuli, as well as locally relax the sphincters with microdoses of drugs.
What was shown on animals
- Esophagus: The implant produced "swallow waves" without actual swallowing, including controlled relaxation of the lower esophageal sphincter (via micro-delivery of glucagon), and programmable forward/retrograde waves—essentially a peristaltic "joystick."
- Stomach. After 20 minutes of stimulation, the frequency of peristalsis increased approximately twofold compared to the control (n≈4, p<0.05).
- Metabolic "illusion of satiety". In fasting conditions, 30-minute stimulation (esophagus or stomach) led to hormonal shifts: an increase in GLP-1 and insulin, a decrease in ghrelin (appetite hormone); with gastric stimulation, an increase in glucagon was also noted. The profile as a whole resembled the postprandial state.
Safety and engineering details
Short in vitro biocompatibility tests (material extracts) showed no toxicity; in vivo 7 days after implantation - normal wall extensibility and no device migration/gross tissue damage. (Further durability and reliability require long-term testing.)
Why is this necessary?
- Dysmotility and refractory conditions. Achalasia, gastroparesis, dysphagia, postoperative disorders - where classic drugs/operations often give an incomplete effect. Local multichannel stimulation is closer to real physiology than existing "single-channel" open-loop implants.
- Metabolic disorders. By controlling the gut-brain afferent pathways, the device could potentially modulate appetite and metabolism, which is interesting for obesity/diabetes (hypothesis so far, no evidence in humans).
Limitations and what's next
This is preclinical work on pigs, in the acute-subacute mode. Ahead are long-term studies on contact stability, energy supply, risk of fibrosis, precise stimulation protocols, and then early clinical trials on patients with severe forms of dysmotivity. But it has already been shown that peristalsis can be “switched on” on command, and hormonal responses can be shifted towards satiety - all through endoscopic access.
