New publications
LM11A-31 drug slows progression of Alzheimer's disease in trial
Last reviewed: 02.07.2025

All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.

In a recent study published in the journal Nature Medicine, scientists conducted a randomized, double-blind, placebo-controlled phase 2a study to examine the safety and efficacy of LM11A-31 in the treatment of Alzheimer's disease (AD) through modulation of the p75 neurotrophin receptor (p75NTR).
Late-onset AD is the most common form of dementia, characterized by synaptic failure, degeneration, and loss of nerve cells. Although the two leading drugs for the treatment of AD target the accumulation of abnormal amyloid-β or tau proteins, they only address part of the pathophysiology. Another approach involves targeting receptors and signaling networks that influence fundamental biological pathways. Preclinical studies show that modulation of p75NTR with a novel small chemical molecule, LM11A-31, reduces synaptic loss caused by amyloid and abnormal tau.
Description of the study
In this randomized clinical trial, the researchers examined whether LM11A-31 could slow the progression of Alzheimer's disease by modulating p75NTR in humans.
Study participants were given LM11A-31 oral capsules at doses of 200 mg and 400 mg or placebo in a 1:1:1 ratio to 242 patients with mild to moderate asthma for 26 weeks. Participants had biologically confirmed Alzheimer's disease (cerebrospinal fluid amyloid β protein 42 (Aβ42) level below 550 ng/L or Aβ42:β40 ratio below 0.89) diagnosed according to McKhann criteria, with Mini-Psychiatric Examination (MMSE) scores of 18 to 26, Geriatric Depression Scale (GDS) scores below 5.0, modified Hachinski Ischemic Scale (HIS) scores ≤ 4.0, formal education ≥ 8 years, and previous cognitive decline ≥ 6 months.
Eligible participants had taken acetylcholinesterase inhibitors (AChEIs) or partial NMDA receptor antagonists for ≥ 3 months prior to study entry. They did not take illicit drugs such as antipsychotics, benzodiazepines, antiepileptic drugs, sedatives, centrally active antihypertensives, nootropics (except ginkgo biloba), or opioid-containing analgesics.
The primary outcome of the study was safety and tolerability, assessed using the Columbia Suicidal Thoughts and Behavior Severity Rating Scale (C-SSRS), vital signs, blood pressure, and hematological parameters. Structural magnetic resonance imaging (cMRI), fluorodeoxyglucose positron emission tomography (FDG-PET), and cerebrospinal fluid (CSF) biomarkers were used to assess secondary cognitive outcomes. AD measures included Thr181-phosphorylated tau, total tau protein, Aβ40, Aβ42, and AChE activity. The team used a customized neuropsychological test to assess secondary cognitive outcomes at baseline, weeks 12, and 26.
Research results
The study found LM11A-31 to be safe and well tolerated, with no significant safety concerns. The most common adverse events included headache, diarrhea, eosinophilia, and nasopharyngitis, with gastrointestinal problems and eosinophilia being the leading reasons for discontinuation. There were more discontinuations in the 400 mg group compared with the 200 mg and placebo groups. MRI revealed no safety concerns, including amyloid-related abnormalities. There were no significant differences in cognitive scores or amyloid abnormalities between the two treatment groups.
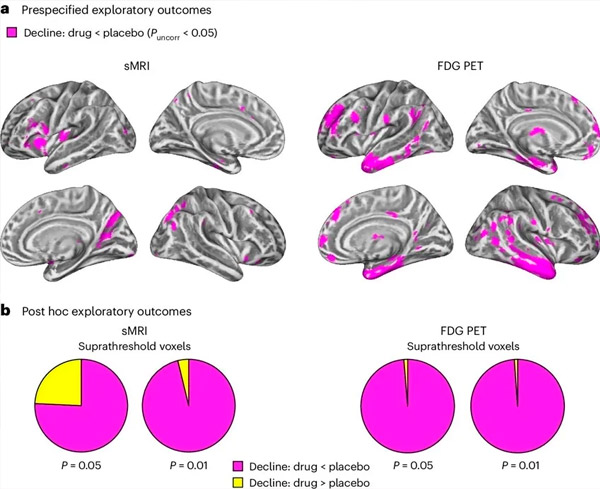
A. Two-way mixed models analysis of covariance examined interactions between treatment (drug or placebo) and time (pre- or post-treatment). A one-tailed t-contrast examining the interaction hypothesis (drug slows progression compared with placebo) showed that LM11A-31 treatment slowed longitudinal degeneration (left panels) and glucose hypometabolism (right panels) in the drug group (cMRI, n = 127; PET, n = 121) compared with the placebo group (cMRI, n = 66; PET, n = 62). Voxels showing this interaction are shown at an uncorrected threshold of P < 0.05 (magenta) on a population-specific cortical surface. Left and right hemispheres are shown in the top and bottom rows, respectively. Brain regions showing interactions inconsistent with the hypothesis are shown in Figure 7 in the Supplementary Data.
B. Total number of voxels in predefined vulnerable AD brain regions (total area of pie charts) showing either an interaction in line with the hypothesis (magenta) or an interaction inconsistent with the hypothesis (yellow) in each imaging modality (cMRI, left panel; FDG PET, right panel) at increasingly liberal thresholds of uncorrected P < 0.01 and P < 0.05. Monte Carlo simulations showed that the ratios of voxels showing effects in line with the hypothesis versus inconsistent with the hypothesis were significantly higher than those observed based on randomly generated data for both cMRI and PET (P < 0.001 for each modality; two-tailed test).
LM11A-31 effectively reduced the increase in CSF Aβ42 and Aβ40 compared to the placebo group. The drug also showed a reduction in the median annual percentage change in the presynaptic protein biomarker SNAP25 and a decrease in the postsynaptic biomarker NG, indicating a slowing of the loss of presynaptic and postsynaptic connections. LM11A-31 also reduced the increase in YKL40, leading to a decrease in MMSE scores and an increase in ADAS-Cog-13 scores. The drug also reduced gray matter loss in the frontal lobe and posterior parietal cortex and a decrease in glucose metabolism in areas such as the entorhinal cortex, temporal cortex, hippocampus, insular cortex, and prefrontal cortex.
Conclusion
The study concluded that modulation of p75NTR by LM11A-31 is suitable for larger clinical trials. LM11A-31 met the primary safety endpoint and was well tolerated in patients with mild to severe AD. The results indicate the need for further studies with longer treatment durations to evaluate the potential of small molecules to regulate p75NTR as a disease-modifying therapy in AD. The study showed that LM11A-31 significantly affected several biomarkers, including Aβ40, Aβ42, SNAP25, NG, and YKL40, indicating a slowing of pathological progression. Future studies may evaluate additional indicators of glial health.
