Medical expert of the article
New publications
FDA investigates efficacy of rapid tests for home HIV diagnosis
Last reviewed: 01.07.2025

All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.
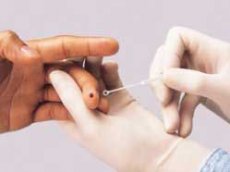
US regulators have begun evaluating the effectiveness of home HIV diagnostic testing. Experts at the US Food and Drug Administration (FDA) are examining the products from OraSure Technologies.
OraQuick test strips are designed to detect carriers of the immunodeficiency virus. Blood or plasma, as well as saliva, can be used as a substance for conducting the study. 20 minutes after the strip comes into contact with the biological material, it will show the presence or absence of antibodies to HIV in the sample being tested.
According to the FDA, medical professionals have been using OraQuick test systems since 2004. The accuracy of diagnosing HIV infection or its absence when used professionally is 99%. OraSure Technologies plans to offer its own products for mass use. In order to sell the test strips freely, the manufacturer must obtain approval from the regulatory agency.

However, in a human volunteer test, the OraQuick was 93 percent accurate in diagnosing HIV. The FDA requires that the test be at least 95 percent accurate. The agency estimates that this could lead to approximately 3,800 people being misled into believing they were HIV-negative.
The manufacturer, in turn, claims that the free sale of express tests, for the purpose of which the assistance of medical workers is not required, will make it possible to examine a significant part of the population. For every million Americans examined, the company expects to find approximately 9 thousand carriers of the immunodeficiency virus.


 [
[