New publications
FDA Clears First Cream-Formed Treatment for Chronic Hand Eczema
Last reviewed: 27.07.2025

All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.
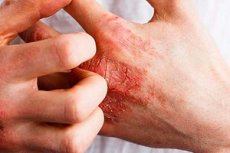
The U.S. Food and Drug Administration (FDA) has approved the first-ever cream specifically designed to treat chronic hand eczema (CHE).
CHE is a common condition characterized by red, itchy, cracked skin on the hands and wrists.
Anzupgo (delgocitinib cream) is approved for adults with moderate to severe CHE who cannot use topical steroids or for whom they are ineffective.
“The approval of Anzupgo confirms our commitment to investing in difficult-to-treat skin diseases to bring new treatments to patients where the need is greatest,” Christophe Bourdon, CEO of LEO Pharma, the maker of Anzupgo, said in a press release.
Unlike atopic dermatitis, the most common form of eczema, CHE is a rare and debilitating condition. It affects about 10% of the U.S. population and lasts more than three months or flares up at least twice a year, according to the National Eczema Association.
Anzupgo works by blocking JAK enzymes, which cause inflammation that triggers flare-ups of hand eczema.
Genetic predisposition, as well as exposure to irritants and allergens, can lead to the development of this form of eczema. People at higher risk are those who work in industries such as cleaning, hairdressing and healthcare, where they are more likely to come into contact with chemicals and react to them.
Research shows that this disease has a serious impact on a person's quality of life and mental health.
A Detroit dermatologist praised the FDA's decision.
“In my time as a dermatologist, I have seen firsthand how much patients suffer from the itching and pain associated with CHE and how much difficulty they have in their daily lives,” said Dr. Linda Stein Gold, director of clinical research at Henry Ford Health in Detroit, in a LEO Pharma U.S. press release. “I believe this new treatment option will be welcomed by dermatologists seeking effective and safe ways to manage these symptoms.”
Studies conducted before FDA approval showed that people with hand eczema who used the cream experienced improvement more often than those who used a placebo or dummy cream. The drug does not carry the black box warning that is required for other topical and oral JAK inhibitors.
“We are thrilled that the FDA has recognized the impact moderate to severe chronic hand eczema has on patients,” said Christine Belleson, CEO and president of the National Eczema Association, in a press release.
“For people living with this debilitating skin condition on their hands, it is extremely difficult; it impacts their ability to work, touch others and connect with the people who matter to them,” she added. “This endorsement brings hope and perspective to the eczema community and those seeking lasting relief from devastating symptoms.”
The cream has already been approved in the European Union, the UK, Switzerland and the United Arab Emirates.
