New publications
Celiac disease: new evidence on the effects of gluten
Last reviewed: 02.07.2025

All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.

Today is World Celiac Day. Celiac disease is a chronic autoimmune disease that affects about 1% of the world's population. It is caused by consuming gluten proteins from wheat, barley, rye and some types of oats. A gluten-free diet protects patients with celiac disease from serious intestinal damage.
Together with colleagues, chemist Dr. Veronica Dodero from the University of Bielefeld was able to determine new details of how certain molecules derived from gluten cause leaky gut syndrome in celiac disease.
The key finding of the study: a certain protein fragment produced in active celiac disease forms nanostructures called oligomers and accumulates in the model intestinal epithelial cells. The technical name for this molecule is 33-mer deamidated gliadin peptide (DGP). The research team found that the presence of DGP oligomers can open the tightly closed lining of the intestine, leading to leaky gut syndrome.
The study was published in the journal Angewandte Chemie International Edition.
Wheat Peptides That Cause Leaky Gut
When we eat wheat, our bodies are unable to completely break down the gluten proteins. This can lead to the formation of large gluten fragments (peptides) in our intestines. In cases of active celiac disease, researchers have found that an enzyme called tissue transglutaminase 2 (tTG2), present in humans, modifies a specific gluten peptide, resulting in the formation of 33-mer DGP. This usually occurs in a part of our intestines called the lamina propria. However, recent studies have shown that this process can also occur in the intestinal lining.
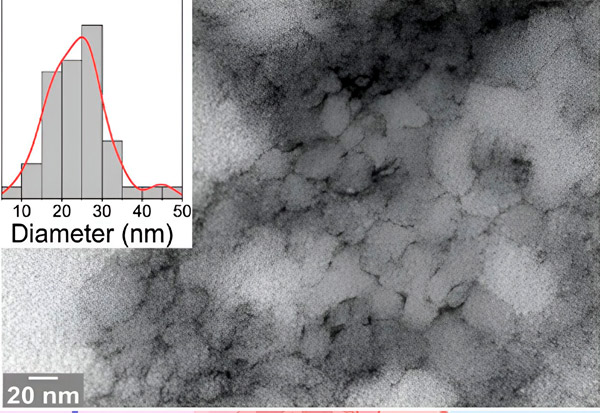
An electron micrograph from the study shows the problematic peptide 33-mer DGP with sharp structures that can open the intestinal barrier. Source: Bielefeld University
"Our interdisciplinary team characterized the formation of 33-mer DGP oligomers using high-resolution microscopy and biophysical methods. We found increased permeability in a model of intestinal cells upon accumulation of DGP," said Dr. Maria Georgina Herrera, first author of the study. She is a researcher at the University of Buenos Aires in Argentina and was a postdoctoral fellow at the University of Bielefeld.
When the intestinal barrier is disrupted
Leaky gut syndrome occurs when the lining of the intestines becomes permeable, allowing harmful substances to enter the bloodstream, leading to inflammatory responses and various diseases. In the case of celiac disease, there is debate about the early stages of increased permeability. The leading theory is that chronic inflammation in celiac disease leads to leaky gut.
However, there is a second theory that suggests that gluten's effects on the cells of the intestinal lining are the root cause. According to this view, gluten directly damages the cells of the intestinal lining, making them permeable, causing chronic inflammation and potentially leading to celiac disease in susceptible individuals.
However, since gluten is consumed daily, what are the molecular triggers that lead to leaky gut in patients with celiac disease? If oligomers of 33-mer DGP are formed, they can damage the epithelial cell network, allowing gluten peptides, bacteria, and other toxins to leak en masse into the bloodstream, leading to inflammation and, in the case of celiac disease, an autoimmune response.
"Our findings strengthen the medical hypothesis that the disruption of the epithelial barrier caused by gluten peptides is the cause and not the result of the immune response in patients with celiac disease," says lead author Dr. Veronica Dodero from the Department of Chemistry at Bielefeld University.
Link between 33-mer DGP and celiac disease
Human leukocyte antigens (HLA) are proteins found on the surface of cells in the body. They play an important role in the immune system, helping it distinguish between self-cells and foreign substances such as bacteria or viruses.
In the case of celiac disease, two specific HLA proteins, namely HLA-DQ2 and HLA-DQ8, are strongly associated with the disease. The 33-mer DGP fits perfectly with HLA-DQ2 or HLA-DQ8 and triggers an immune response that leads to inflammation and atrophy of the villi of the small intestine. This strong interaction turns DGP into what scientists call a superantigen. For those suffering from celiac disease, a gluten-free diet is the only lifelong therapy.
